Protein family linked to autism suppresses the development of inhibitory synapses
Synapse development is promoted by a variety of cell adhesion molecules that connect neurons and organize synaptic proteins. Many of these adhesion molecules are linked to neurodevelopmental disorders; mutations in neuroligin and neurexin proteins, for example, are associated with autism and schizophrenia. According to a study in The Journal of Cell Biology, another family of proteins linked to these disorders regulates the function of neuroligins and neurexins in order to suppress the development of inhibitory synapses.
Like neurexins and neuroligins, the neuronal proteins MDGA1 and MDGA2 have been linked to autism and schizophrenia, but their function in neurodevelopment was unknown. Both MDGA proteins localize to the plasma membrane, and their extracellular domains are similar to those of cell adhesion molecules. On the other hand, postsynaptic neuroligin proteins are known to help synapses form by associating with neurexins on presynaptic membranes. Neuroligin-2 specifically boosts the development of inhibitory synapses, whereas neuroligin-1 promotes the development of excitatory synapses.
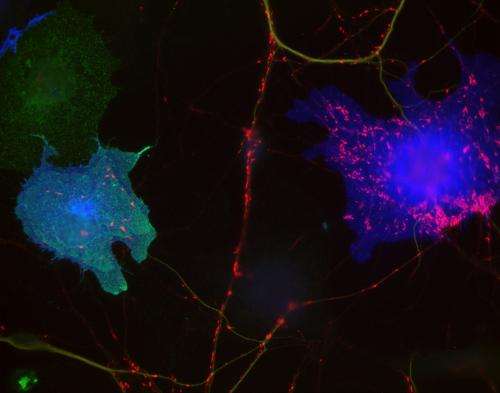
Ann Marie Craig and colleagues from the University of British Columbia investigated the function of MDGAs using co-culture assays, in which postsynaptic proteins like neuroligin-1 or -2 are expressed in non-neuronal cells and then tested for their ability to induce presynaptic differentiation in neighboring neurons. MDGA1 didn't promote synapse formation in these assays. Instead, it inhibited the ability of neuroligin-2 to promote synapse development. The researchers found that MDGA1's extracellular domains bound to neuroligin-2, blocking its association with neurexin. The same domains were sufficient to inhibit neuroligin-2's synapse-promoting activity. In contrast, MDGA1 didn't show high affinity binding to, or inhibit the function of, neuroligin-1. This suggested that, by inhibiting neuroligin-2, MDGA1 might specifically suppress the development of inhibitory synapses, so Craig and colleagues investigated MDGA1 function in cultured hippocampal neurons.
"Overexpressing MDGA1 in neurons reduced the density of inhibitory synapses without affecting excitatory synapses," Craig says. Knocking down MDGA1, on the other hand, increased inhibitory synapse development but had no effect on excitatory synapses.
"I can't think of any other proteins that specifically suppress inhibitory synapse formation," says Craig. Indeed, very few proteins in general have been identified as negative regulators of synapse development, compared to the many proteins that are known to promote synaptogenesis. The results suggest that function-altering mutations in the MDGA proteins may disrupt the balance of excitatory and inhibitory synapses in the brain, potentially explaining the development of autism and other neurodevelopmental disorders.
"This puts MDGAs in the same pathway as neurexins and neuroligins and strengthens the evidence for the involvement of synaptic organizing proteins in autism and schizophrenia," Craig explains. As well as investigating the function of MDGA2, the researchers want to explore the therapeutic potential of MDGA1 inhibitors, not only against autism and schizophrenia but also for the treatment of epilepsy, in which excitatory and inhibitory synapses are also imbalanced
More information: Pettem, K.L., et al. 2013. J. Cell Biol. doi:10.1083/jcb.201206028