First vaccine against four flu strains ready to ship
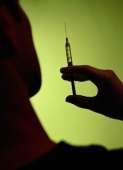
(HealthDay)—The U.S Food and Drug Administration has approved shipping of the first vaccine to protect against four strains of seasonal flu.
The GlaxoSmithKline vaccine, called Fluarix Quadrivalent, was approved by the FDA last December for use in adults and children aged 3 and older. But approved flu vaccines still have to be certified by the FDA each season before they can be shipped to health care providers.
Viruses that cause seasonal flu are classified as A or B strains. Most current flu vaccines provide protection against three strains: the two A strains most common in people, and the B strain expected to be predominant in an upcoming flu season.
This is the first season that vaccines protecting against more than three flu strains will be available.
"Trivalent [three-strain] influenza vaccines offer important protection against influenza. But since the late 1980s, scientists noted that two B virus lineage strains circulate to varying degrees each year, and it's difficult to predict which one will cause the most illness in a particular influenza season. Fluarix Quadrivalent addresses this by protecting against both B strains," Dr. Leonard Friedland, vice president of scientific affairs and public policy at GlaxoSmithKline Vaccines, North America, said in a company news release.
The U.S. Centers for Disease Control and Prevention placed the largest order for Fluarix Quadrivalent—more than 4 million doses—and will distribute those doses to state and local health care providers.
GlaxoSmithKline estimates it will provide up to 10 million doses of quadrivalent influenza vaccine in the United States, and 22 million to 24 million doses of influenza vaccine overall. Fluarix Quadrivalent also is approved in Germany and the United Kingdom.
In clinical trials of the new vaccine, the most common negative side effects in adults were pain at the injection site, muscle aches, headaches and fatigue. Negative side effects among children included: pain at the injection site, redness and swelling, drowsiness, irritability, loss of appetite, fatigue, muscle aches, headache and gastrointestinal symptoms.
More information: The U.S. Centers for Disease Control and Prevention has more about the seasonal flu vaccine.
Health News Copyright © 2013 HealthDay. All rights reserved.