Two forms of Parkinson's disease identified
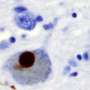
Why can the symptoms of Parkinson's disease vary so greatly from one patient to another? A consortium of researchers, headed by a team from the Laboratoire CNRS d'Enzymologie et Biochimie Structurales, is well on the way to providing an explanation. Parkinson's disease is caused by a protein known as alpha-synuclein, which forms aggregates within neurons, killing them eventually. The researchers have succeeded in characterizing and producing two different types of alpha-synuclein aggregates. Better still, they have shown that one of these two forms is much more toxic than the other and has a greater capacity to invade neurons. This discovery takes account, at the molecular scale, of the existence of alpha-synuclein accumulation profiles that differ from one patient to the next. These results, published on October 10 in Nature Communications, represent a notable advance in our understanding of Parkinson's disease and pave the way for the development of specific therapies targeting each form of the disease.
Parkinson's disease, which is the second most frequent neurodegenerative disease after Alzheimer's, affects some 150,000 people in France. According to those suffering from the disease, it can manifest itself in the form of uncontrollable shaking (in 60% of patients) or by less-localized symptoms such as depression, behavioral and motor disorders. These differences in symptoms point to different forms of Parkinson's disease.
This condition, for which no curative treatment currently exists, is caused by the aggregation in the form of fibrillar deposits of alpha-synuclein, a protein that is naturally abundant at neuron junctions. These misfolded alpha-synuclein aggregates propagate between neurons. When they invade a new neuron, they are capable of recruiting normal alpha-synuclein and adding it to the deposit. For this reason, many researchers advocate that the alpha-synuclein of the aggregates should be considered as an infectious protein, in other words a prion. Highly toxic, the alpha-synuclein deposits end up by triggering a process of apoptosis, i.e. cell death.
The researchers have shown that there is not just one single type of aggregate. They succeeded in producing two types of aggregate that only differ in how the protein stacks up. At the millionth of a millimeter scale, the first form of aggregate resembles spaghetti, whereas the second form is long and flat, recalling the shape of wider pasta such as linguine. The team of scientists then tried to determine whether these structural differences result in functional differences. To find out, they placed the two types of aggregates in contact with neuronal cells in culture. They discovered that the capacity of the "spaghetti" form to bind to and penetrate cells is notably greater than that of the "linguine" form. The "spaghetti" form is also considerably more toxic and rapidly kills the infected cells. This form has shown itself to be capable of resisting the cell mechanisms responsible for eliminating it, whereas the "linguine" form is, to a certain extent, controlled by the cell.
The researchers are convinced that the existence of at least two forms of alpha-synuclein aggregates explains why doctors are faced with different Parkinson's diseases depending on the patient. Experiments on mice are currently underway to confirm this hypothesis. Furthermore, the scientists consider that analysis of the type of aggregate could lead to an efficient diagnosis method, which would make it possible in particular to assess the virulence of the disease for each patient. Finally, they hope that by refining the characterization of the structure of the aggregates, it will be possible to develop targeted therapeutic strategies for each variant in order to slow down the propagation of abnormal alpha-synuclein within the brain.
More information: Bousset, L. et al. Structural and functional characterization of two alpha-synuclein strains, Nature Communications, 10 October 2013. DOI: 10.1038/ncomms3575