A trigger for muscular diseases
A University of Arizona doctoral candidate has shown for the first time that genetic mutations in the titin gene can cause skeletal muscle myopathy, a disease in which muscle fibers do not function properly, resulting in muscle weakness. Myopathic disease can affect heart muscles as well as skeletal muscles, and titin is responsible for many problems associated with heart disease.
The research was done by Danielle Buck, a doctoral candidate in the UA's Department of Molecular and Cellular Biology. She worked under the direction of Henk Granzier, a professor in cellular and molecular medicine and physiology, who has studied titin for years.
Previous studies had shown that alterations in titin are involved in muscular myopathies, but whether these deviations actually cause myopathies, or merely result from them, has remained a mystery.
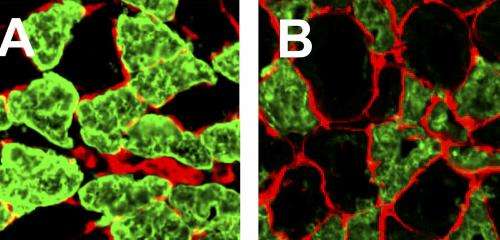
Buck has shown that mutations in the titin gene do in fact cause myopathies in skeletal muscles. Her study, published today in the Journal of General Physiology, could be an important first step in developing treatments to address causes of the disease.
"Patients with muscle myopathy experience muscle weakness, but not a lot has been known about what is going wrong at the molecular and genetic level, except that titin is often involved," Buck said. "Many patients with heart disease also have mutations in titin. So to develop treatments we need to understand the structure of titin and how it can cause or respond to disease."
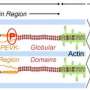
"With about 35,000 amino acids, titin is the largest protein known, roughly 100 times larger than typical proteins, which have only around several hundred amino acids," Granzier explained. Amino acids are the building blocks of proteins.
Titin, he said, functions as a molecular spring that makes tissues elastic so that when they deform they can snap back again. "Titin is a vital determinant of the elasticity of skeletal and heart muscles, which is very important for normal muscular function," he noted.
"Titin is like the stretchy material in a rubber balloon," said Buck. "If you have a balloon that is too stretchy or too stiff, then it's not going to be able to expand or contract. Tissues also need to have elasticity so that they can restore their original shape after they have been contracted."
Conducting genetic testing for mutations in the titin gene and studying the defects in the protein have been challenging due to titin's "enormous size," Granzier said. "But excellent facilities at the University of Arizona have enabled researchers to make great impact and progress has recently accelerated."
Buck's research "has directly shown that introducing specific changes to the titin gene can lead to disease in skeletal muscles," Granzier said. "We know now that titin itself can trigger the disease. Danielle's research shows that this giant protein needs to be tuned just right or it can cause myopathies to develop in skeletal muscles."
Buck's research "also demonstrated for the first time that changing a part of the gene results in a cascade of additional damaging changes in the protein," he added.
"We found that in skeletal muscles, deleting one area of titin can affect expression of the entire protein and other areas can subsequently be deleted as well," Buck said. "Shortening titin leads to a cascade of effects that cause titin to be even shorter, and that causes the muscle to become very stiff."
Buck approached her work from many levels, Granzier said. "She worked at the gene level, the transcription level, the protein level and the functional level of cells and tissues to get an integrative understanding of the changes that this genetic modification caused."
"We try to look at all these levels so that we can get a deeper understanding of the mechanisms that give rise to disease," he added. "It is a multidisciplinary study, from molecular and cellular biology to integrative physiology."
Understanding what factors cause myopathies could enable researchers to reverse the disease in humans by developing medications to counter damaging activity of the gene, Buck said.
"The next step ideally would be to use this model as an avenue to find new future therapeutic targets," she said.
Buck already has begun to forge into research around a possible cure for myopathies.
More information: Buck, D., et al. 2014. J. Gen. Physiol. DOI: 10.1085/jgp.201311129