Study identifies path to safer drugs for heart disease, cancer
Massachusetts General Hospital (MGH) investigators may have found a way to solve a problem that has plagued a group of drugs called ligand-mimicking integrin inhibitors, which have the potential to treat conditions ranging from heart attacks to cancer metastasis. In a Nature Structural & Molecular Biology paper receiving advance online publication, the researchers provide a structural basis for the design of new and safer integrin inhibitors.
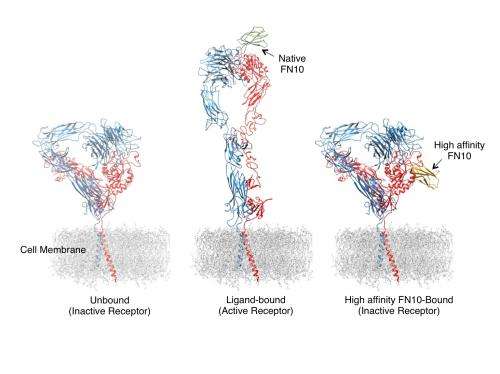
Integrins are receptor proteins found on the surface of cells that determine whether or not cells adhere to adjacent cells and the surrounding extracellular matrix. Under normal circumstances, integrins only become activated – which allows them to bind to other cells or extracellular molecules – in response to specific signals from within the cell. If integrins become overactive, cells become too "sticky" – leading to clogged arteries, pathological inflammation, the excess tissue growth called fibrosis or the spread of cancer. Current drugs developed to inhibit integrin activation by mimicking the shape of ligands – the molecules that interact with receptors – have had unintended effects in some patients, and as a result only a handful have received FDA approval.
"Integrins have an intrinsic ability to shape-shift when they switch from an inactive to an active, adhesive state," explains M. Amin Arnaout, MD, director of the MGH Leukocyte Biology Program and the Inflammation and Structural Biology Program, senior author of the study. "Unfortunately, under some circumstances the integrin inhibitors that have been developed to date can inadventently induce this shape shifting, and use of these drugs have produced serious, sometimes fatal side effects such as excessive bleeding."
In their search for drugs that would not induce these complications, the MGH team focused on an extracellular matrix protein called fibronectin, which binds to an integrin called αvβ3. Their detailed structural analysis of the bond between αvβ3 and various forms of FN10, the fibronectin molecule that interacts with αvβ3, identified a high-affinity version of FN10 that binds more strongly than the common form without causing unintended receptor activation. This first report of the three-dimensional atomic structure of an integrin binding with a ligand-mimicking molecule that does not cause inadvertent activation could enable the design of a new generation of integrin inhibitors without the complications that have limited their application.
More information: Structural basis for pure antagonism of integrin αVβ3 by a high-affinity form of fibronectin, DOI: 10.1038/nsmb.2797