Cancer and the Goldilocks effect: Too much or too little of a single enzyme may promote cancer
Researchers at the University of California, San Diego School of Medicine have found that too little or too much of an enzyme called SRPK1 promotes cancer by disrupting a regulatory event critical for many fundamental cellular processes, including proliferation.
The findings are published in the current online issue of Molecular Cell.
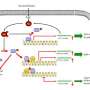
The family of SRPK kinases was first discovered by Xiang-Dong Fu, PhD, professor in the Department of Cellular and Molecular Medicine at UC San Diego in 1994. In 2012, Fu and colleagues uncovered that SPRK1 was a key signal transducer devoted to regulating alternative pre-mRNA splicing, a process that allows a single gene to produce multiple mRNA isoforms, which in many cases encode functionally distinct proteins. In this pathway, SRPK1 was a downstream target of Akt, also known as protein kinase B. Akt- activated SRPK1 moves to the nucleus to induce its targeted splicing factors.
In their latest paper, Fu and colleagues report that SRPK1 was found to act as a tumor suppressor because when ablated or removed from mouse embryonic fibroblasts, unwanted cell transformation occurred. Unexpectedly, when SRPK1 was overexpressed in mouse cells, tumor development also happened.
"To my knowledge, this is the first time it has been shown that a signal kinase behaves as a tumor suppressor or a promoter, depending upon its abundance in the same cell" said Fu. "The point is that too much or too little are both bad."
Such contrary phenomena are due to a surprising role of SRPK1 in regulating the activity of Akt via a specific Akt phosphatase discovered earlier by Alexandra C. Newton, PhD, professor of pharmacology at UC San Diego. The Akt phosphatase cannot find Akt when there is too little SRPK1 to assist, and the phosphatase is tied up when there is too much SRPK1. In both cases, the result is a dampening of Akt inactivation.
As Akt plays a key role in many cellular processes, such as glucose metabolism, apoptosis, proliferation and all key aspects of tumor development, the elucidated mechanism provides a critical insight into tumorigenesis in humans. Indeed, compared to normal cells, many tumors show SRPK1 overexpression while others display reduced expression.
The findings may have future therapeutic implications, but Fu said the challenges remain daunting. "Most tumors show SRPK1 overexpression, so it may be possible to treat certain cancers with a specific SRPK1 inhibitor. This has been already demonstrated by others. But suppressing a cancer not related to SRPK1 overexpression could actually stimulate that cancer."