Study compares structures of Huntington's disease protein
Neutron scattering research at the Department of Energy's Oak Ridge National Laboratory has revealed clear structural differences in the normal and pathological forms of a protein involved in Huntington's disease.
Huntington's disease, an incurable neurodegenerative disorder, starts as a genetic mutation that leads to an overabundance of "huntingtin" protein fragments, which form clumps in the brain.
Valerie Berthelier of the University of Tennessee Graduate School of Medicine, who co-led the study published in Biophysical Journal with ORNL's Chris Stanley, said the goal was to establish a baseline understanding of huntingtin's structure in order to eventually determine the true structural basis of Huntington's disease.
"This is a very first step—the hope is that we do this basic research to shed light on the structures of the protein," Berthelier said. "If we can start identifying any of these structures as toxic or potentially toxic, and then think about how drugs could interact with them, then we might be getting to the point of rationally designing therapeutics that would target those specific structures."
The researchers conducted a side-by-side study of model protein systems in solution using a time-resolved small-angle neutron scattering technique at ORNL's High Flux Isotope Reactor. The use of neutrons, a non-damaging but highly penetrating particle, allowed the team to study the biological materials over time without degrading the samples' structural integrity.
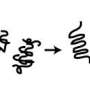
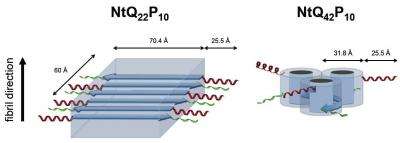
"We compared the normal and disease versions of the protein to see how they change over time," Stanley said. "You can see there's a discrepancy all the way from the early stages to the end-state fibrils."
The study's results showed key differences in the ways mutant and normal huntingtin proteins take shape. The disease protein, for instance, initially forms aggregates of one to two peptides, whereas the normal version makes bigger aggregates, gathering seven or eight peptides together.
These data on the very early stages of protein aggregate formation support a growing focus of the research in the amyloid field. Amyloid disorders, such as Parkinson's, Alzheimer's and Huntington's, all involve protein aggregation phenomena leading to a disease.
"There is no strong correlation between neuronal cell loss and the amount of protein aggregates found in the brain," Stanley said. "You could have a case with a lot of aggregates but minimal symptoms—and you can find the converse. Researchers think there must be something happening at the earliest stage that's giving rise to toxicity."
Stanley says the team hopes to continue its research to obtain higher-resolution structural data and refine their understanding of the huntingtin protein.
"We'd like to use this small-angle neutron scattering technique in combination with others to get a better idea for how these early structures are forming and also ask the question—are they toxic or not?" Stanley said.