Serotonin receptor structure revealed
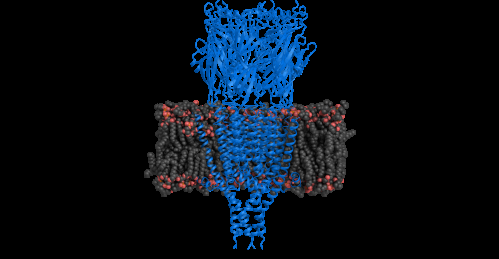
The structure of a serotonin receptor has been completely deciphered for the first time using crystallography. This study, published online in Nature on August 3, 2014, opens the way towards the design of new drugs that might be able to control nausea, one of the main adverse effects of chemotherapy and anesthesia.
This work was carried out by a team at the Institut de Biologie Structurale (CNRS/CEA/Université Joseph Fourier) and the Physical Chemistry of Polymers and Membranes Laboratory (EPFL, Switzerland), working in collaboration with scientists from the Architecture et Fonction des Macromolécules Biologiques (CNRS/Université Aix-Marseille) and Dynamique Structurale des Macromolécules (CNRS/Institut Pasteur) laboratories, and with the company, Théranyx.
Communication between neurons in the brain is assured notably by receptors, i.e. cell membrane proteins, to which neurotransmitters can bind. Serotonin is one of the best known of these neurotransmitters. It is involved for example in the biology of the sleep-wake cycle, pain or anxiety. The serotonin receptor under study forms part of the "Cys-loop" family of receptors, which are extremely important from a pharmacological point of view. This family notably includes the receptors to which nicotine binds, and also the target receptors for antidepressants (such as valium) or general anesthetic agents.
For the first time, scientists have determined the complete structure of a serotonin receptor in a mammal, the mouse, whose receptors are structurally similar to those of humans. Previously, it had only been possible to study receptors in the Cys-loop family using homologs present in certain bacteria. In order to elucidate the complete structure of a serotoninergic receptor, the researchers had to overcome several obstacles, ranging from its production from cell lines cultivated in vitro to its purification and crystallization. They then imaged the protein by X-ray diffraction to reveal the position and organization of each of the amino acids making up its structure.
These findings shed new light on the functioning of this receptor, how it binds serotonin and transmits neural signals. They could pave the way for new drugs that may be able to control nausea, one of the principal adverse effects of chemotherapy and anesthesia. Developing a compound directly based on the structure of the receptor may lead to the rapid discovery of a drug capable of binding to it and forming interactions. This receptor is also a potential target for other disorders that affect the digestive nervous system (such as irritable bowel syndrome) or central nervous system (certain types of depression).
More information: "X-ray structure of the mouse serotonin 5-HT3 receptor." Ghérici Hassaine, Cédric Deluz, Luigino Grasso, Romain Wyss, Menno B. Tol, Ruud Hovius, Alexandra Graff, Henning Stahlberg, Takashi Tomizaki, Aline Desmyter, Christophe Moreau, Xiao-Dan Li, Frédéric Poitevin, Horst Vogel and Hugues Nury. Nature, online on August 3, 2014. DOI: 10.1038/nature13552