FDA OKs new varicose vein treatment

(HealthDay)—A new system to permanently treat varicose veins in the legs by sealing the affected veins with adhesive was approved Friday by the U.S. Food and Drug Administration.
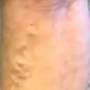
Many people with varicose veins experience no symptoms, while others have mild to moderate pain, blood clots, skin ulcers or other problems.
The VenaSeal system is meant for treatment of varicose veins that cause symptoms and is the first treatment to use an adhesive agent to cut off blood supply to affected veins, the FDA said Friday in a news release.
In the newly approved treatment, a catheter is inserted through the skin into the affected vein. The adhesive is injected through the catheter and hardens into a solid material. Ultrasound is used to monitor placement of the adhesive, the FDA said.
"This new system is the first to permanently treat varicose veins by sealing them with an adhesive, thereby giving patients another treatment option for this common condition," Dr. William Maisel, acting director of the Office of Device Evaluation at the FDA Center for Devices and Radiological Health, said in the news release.
"Because the VenaSeal system does not incorporate heat application or cutting, the in-office procedure can allow patients to quickly return to their normal activities, with less bruising," he added.
The FDA's approval was based on three clinical studies, including one in the United States that compared 108 patients treated with the VenaSeal system and 114 patients treated with radio waves (radio-frequency ablation). The results showed that the VenaSeal system was a safe and effective treatment for varicose veins, the FDA said.
Problems included vein inflammation and burning or tingling in the treatment area.
The system should not be used on patients sensitive to the adhesive, those with acute inflammation of the veins due to blood clots, or those with acute whole-body infection, the FDA said.
The system is made by Covidien LLC, in Morrisville, N.C.
More information: The U.S. National Heart, Lung, and Blood Institute has more about varicose veins.
Copyright © 2015 HealthDay. All rights reserved.