New drug 'dials down' protein synthesis to treat demyelinating diseases
In 2013, University at Buffalo researchers published a paper showing how slowing down protein synthesis can improve myelin production and repair in some demyelinating diseases, such as Charcot-Marie-Tooth disease, (CMT). The research held promise for other misfolded protein diseases, such as Alzheimer's, Parkinson's and amyotrophic lateral sclerosis (ALS).
The drawback was that the small molecule that slowed protein synthesis had side effects rendering it unsuitable for human use.
At the time, the UB researchers, led by Lawrence Wrabetz, MD, director of UB's Hunter James Kelly Research Institute, noted that if they could find a new version of the agent they used that was safe and effective, it could lead to new therapeutic strategies for CMT
Earlier this month, Wrabetz and others co-authored a paper in the journal Science that does just that. The lead author is Anne Bertolotti, program leader in the Medical Research Council (MRC) Laboratory of Molecular Biology, Cambridge, UK.
In preclinical trials in animals, the Science paper reports, a new candidate drug called Sephin1 can markedly improve CMT and familial amyotrophic lateral sclerosis (ALS), two diseases of proteostasis (protein homeostasis, the process keeping protein production in balance in cells).
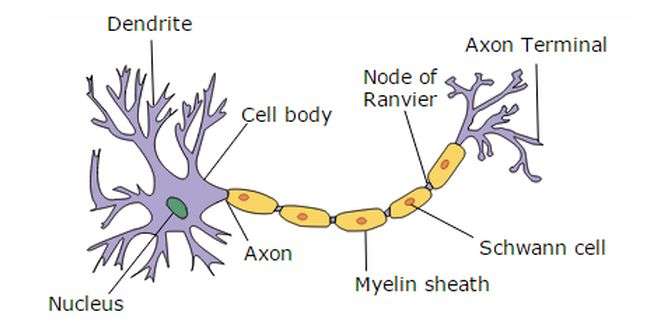
While the diseases are otherwise unrelated, both can result from difficulties in protein folding. In the case of ALS, the misfolded proteins are toxic to neurons and kill them, whereas in CMT neuropathy, the misfolded proteins disturb the production of myelin, the crucial fatty material that normally wraps the axons of neurons, allowing them to signal effectively.
"Our 2013 study and others have found that in diseases caused by proteostasis, keeping protein production more stringently and persistently dialed down is good for limiting disease," said Wrabetz, also professor of neurology and biochemistry in UB's School of Medicine and Biomedical Sciences. "Professor Bertolotti and her colleagues at Cambridge modified a molecule they discovered in 2011 in order to provide a candidate drug that regulates protein production but without the toxic side effects."
Basing the design of the preclinical trial on the same one they published in 2013, Wrabetz and his colleague, Maurizio D'Antonio at San Raffaele Scientific Institute in Milan, joined by Indrajit Das of Cambridge, treated juvenile animals with CMT with Sephin1. After five months of treatment, the results were unequivocally positive.
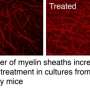
"Motor function in the animals returned to normal, the amount of myelin destruction was reduced by 70 percent and myelin thickness improved remarkably," said Wrabetz.
There were equally positive results when Das treated the familial ALS model with Sephin1 at Cambridge.
Sephin1 regulates a key factor in protein synthesis, and does so by maintaining phosphorylation, or the addition of a phosphate group.
"The finding is important because proteostasis diseases are multiple and affect many people," said Wrabetz, noting that they include neurodegenerative conditions, such as Alzheimer's and Parkinson's, demyelinating diseases such as multiple sclerosis and certain types of cancers and some subtypes of diabetes.
"It's important to emphasize that further studies are necessary to confirm that the effects in these two animal models will translate to patients with CMT and familial ALS and then, that this candidate drug or similar drugs could be useful in other diseases where proteostasis is a factor," Wrabetz explained. "Nonetheless, this study is an important first step toward developing a therapeutic strategy for these diseases with a candidate drug that could potentially be used in clinical trials."
Wrabetz and Laura Feltri, MD, UB professor of biochemistry and neurology, are also interested in exploring the study's relevance to leukodystrophies, the rare and severe developmental diseases of myelin in the brain and nerves. He noted that imbalances in proteostasis have been reported to contribute to some leukodystrophies.
Currently they are exploring whether altered proteostasis is present in animal models of Krabbe leukodystrophy, the disease that afflicted Hunter James Kelly, for which the Hunter James Kelly Research Institute is named.
More information: "Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit." Science 10 April 2015: Vol. 348 no. 6231 pp. 239-242 DOI: 10.1126/science.aaa4484