Is UK evaluation of reproductive tech a model for US?

When the United Kingdom resoundingly approved mitochondrial replacement therapy in February, it became the first country to give people this new medical option. In parallel it gave the United States serious cause to reflect on how it handles matters of reproductive innovation, argues a trio of experts in the journal Science.
"We have fundamentally different regulatory cultures," said co-author Dr. Eli Adashi, former dean of medicine and biological sciences at Brown University.
The essay's other authors are I. Glenn Cohen of Harvard law and ethicist Julian Savulescu of the University of Oxford.
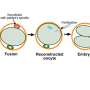
MRT was conceived to prevent a particular set of health problems from continuing in a family's lineage. The mitochondria are structures within cells that help them produce the energy they need. They have their own genome, separate from the DNA in the cell's nucleus that constitutes the vast majority of the genetic code that makes us who we are. Nevertheless, flaws in mitochondrial DNA can result in certain diseases.
The idea of MRT, therefore, is to get the flawed mitochondria out of the picture when a potential child is just one cell: either an egg or an embryo. In MRT, scientists propose to transplant the nucleus of an egg or zygote into a similar cell from a donor who has healthy mitochondria. The UK is now free to investigate in clinical trials whether resulting babies will thrive, as hoped, with the natural blend of their parents' nuclear DNA, but with the mitochondria of a donor.
Different paths
In the United Kingdom, MRT underwent the evaluation of a specialized, tightly focused institution known as the Human Fertilisation and Embryology Authority. The United Kingdom established HFEA in the wake of its experience grappling with in vitro fertilization, a procedure with scientific and ethical similarities to MRT. HFEA's process, which took about four years, ensured a thorough scientific and ethical review as well as public comment on MRT prior to Parliament's approval on Feb. 3, 2015.
The U.S. experience offers many contrasts, the authors note. Foremost is that there is no specialized body for reviewing these kinds of procedures, although the Food and Drug Administration has asserted jurisdiction over MRT via its Office of Cellular, Tissue, and Gene Therapies of the Center for Biologics Evaluation and Research.
"The FDA is a really broad umbrella that is non-specialized," Adashi said. "In the U.K. it's a highly specialized agency that does nothing else but look into matters of reproduction."
The relevant FDA advisory committee first met in February 2014, and then in September the FDA commissioned an ad hoc committee of the Institute of Medicine to consider the ethical and social policy implications of MRT before its proceeds any further. The IOM is not expected to report for another year.
Other differences include that the United Kingdom simply started considering MRT earlier. Reproductive technologies involving embryos are more legally constrained and ethically controversial in the United States, and although American and British scientists both contributed significantly to the development of MRT, the British in particular regard the breakthrough as a point of national pride, the authors wrote.
It's not just about MRT
Whether or whenever the United States eventually approves MRT, there are also other technologies unique to reproduction and heritability on the policy agenda, the authors noted. These include germline editing, in which scientists could hypothetically edit the nuclear DNA of a sperm, egg, or embryo, and generating eggs or sperm from stem cell-derived somatic cells. In this context of several pending reproductive technology issues, the authors argue that the United States may need to devote the same specialized regulatory resources to the questions as the United Kingdom has.
"This examination of the different approaches taken to the regulation of MRT in the U.K. and the U.S. leads us to reexamine the wisdom of burdening the FDA with the regulatory adjudication of MRT as opposed to adopting a HFEA-like paradigm," the authors wrote. Adashi said MRT in the United States is a test case that can benefit from considering the United Kingdom's successful process.
"How we handle MRT will determine how we handle future breakthrough reproductive technologies," Adashi said.
More information: Trio contrast approaches taken by Britain versus the US concerning mitochondrial replacement therapy: medicalxpress.com/news/2015-04 … n-mitochondrial.html