April 10, 2015 report
Trio contrast approaches taken by Britain versus the US concerning mitochondrial replacement therapy
(Medical Xpress)—Glenn Cohen, with Harvard University, Julian Savulescu, with the University of Oxford and Eli Adashi with Brown University have together written and published a Perspectives piece in the journal Science, describing the differing approaches being taken regarding mitochondrial replacement therapy (MRT) in the U.S. versus Britain, and offer their opinion on how things should change in the U.S.
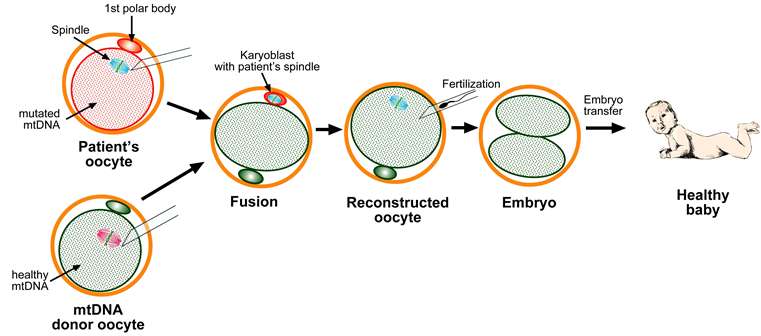
MRT was developed as a means to help prevent mutant mitochondrial syndromes in babies—it involves taking out a defective part of the mitochondrial DNA in a fertilized embryo and replacing it with healthy mitochondrial DNA from a donor. This has led to it sometimes being described as a way for a child to have three biological parents and as a form of abortion because part of the embryo is destroyed in the process. In their article, the three authors outline the history of research and regulation surrounding MRT, and highlight what they see as positive progress in the U.K. (where it was recently legalized) and slow progress in the U.S. This can be ascribed to four main causes they propose: The way it is regulated, abortion issues and, pubic versus private debate and national pride.
In the U.K. MRT is regulated by a special agency called the Human Fertilization and Embryology Authority (HFEA)—their charter is to review the effectiveness and safety of the procedure In the U.S. on the other hand, regulation falls to a division within the FDA called the Office of Cellular, Tissue and Gene Therapies of the Center for Biologics Evaluation and Research, which has thus far seemed to lean towards classifying MRT as a therapy that must pass clinical trials before being used, none of which have ever been considered by the group. The authors also believe that ethical issues related to abortion are also holding up MRT research in the U.S. They also suggest that public opinion and national pride are playing a role—the British people speak proudly of the work done by research facilities in their country and do not seem to see a connection between MRT and abortion—they have also played a role in debating the issues that surround the technology. Meanwhile, in the U.S., public debate has been minimal and talk of abortion has been magnified by the press.
The authors conclude by suggesting that perhaps it is time to move oversight in the U.S. from the FDA to a new authority and to perhaps begin a national conversation on the procedure, as it appears that MRT is coming, whether it happens in the U.S. or not.
See also: Is UK evaluation of reproductive tech a model for US?
More information: Transatlantic lessons in regulation of mitochondrial replacement therapy, Science 10 April 2015: Vol. 348 no. 6231 pp. 178-180. DOI: 10.1126/science.aaa8153
Abstract
Mutant mitochondrial DNA (mtDNA) gives rise to a broad range of heritable clinical syndromes (1). A cure for those affected remains out of reach (1). However, recently developed mitochondrial replacement therapy (MRT) has raised the prospect of disease-free progeny for women carriers (2–4). Moreover, the feasibility of replacing mutant oocytic or zygotic mtDNA with a donated wild-type counterpart in humans has now been firmly established (2–4). In the United Kingdom, legislation regulating the clinical application of MRT, now 10 years in the making, has recently been approved by the House of Commons (5) and the House of Lords (6). The regulatory vetting of MRT in the United States, under way for a year, remains a work in progress (7). Here, we compare and contrast the regulatory history of MRT in the United Kingdom and the United States and examine potential lessons learned.
© 2015 Medical Xpress