Why do healthy people have harmful mutations?

"NIH researchers pilot predictive medicine by studying healthy people's DNA," read the headline of a news release yesterday.
The news release, about intriguing new findings from the ClinSeq program at the National Human Genome Research Institute, states that researchers "sequenced the genomes" of 951 "healthy participants." 103 of the people had disease-associated mutations, and of 79 of them followed up clinically, 34 indeed had the diseases their genotypes predicted, but apparently hadn't known it. More intriguing were the 43 participants who had disease-causing genotypes, but were healthy.
That's certainly news, but it doesn't quite echo the paper it describes, published in The American Journal of Human Genetics.
"The participants were 45 to 65 years of age at enrollment and were selected for a range of atherosclerosis phenotypes," the paper states. The researchers factored in the abnormal lipid profiles. The paper also clearly states that exomes (the 2% or so of the genome that encodes protein) were considered, not full genomes, and scanned for known, dominant mutations that cause "loss-of-function." And the focus on adults eliminates the many single-gene disorders that start in childhood.
So the study isn't quite the grab-bag of average Jills and Joes that the news release suggests. But the results are compelling, and important to the precision medicine effort. They provide powerful evidence for concepts from classical genetics.
Beyond Mendel'S Laws
Chapter 5 in all eleven editions of my human genetics textbook, "Beyond Mendel's Laws," addresses the complexities behind the effects of mutations.
To start, 3 words describe single genes:
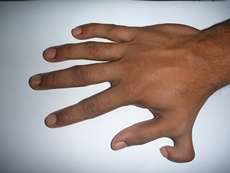
- Penetrance. A person who has a genotype but not the corresponding phenotype (trait or illness) is "non-penetrant," and the gene "incompletely penetrant." A mom with 10 fingers and 10 toes whose child and parent have extra fingers and toes (polydactyly) is non-penetrant. The mom would have to have the mutation to pass it to her child.
- Expressivity. People vary in their experience of the same disease. A child with sickle cell disease may require frequent hospitalization, while his sibling, who has the disease too, does not.
- Pleiotropy. Single-gene diseases may have many symptoms, and different people have different subsets of them. Or, a person may have a symptom not known to be part of a disease, but it really is. This "atypical presentation" is the case for Nicholas Volker, one of the first patients with an undiagnosed disease to get an answer through exome sequencing. The boy's dissolving intestines were due to XIAP deficiency, an immune disorder not previously known to have this symptom.
Interacting genes
When considering more than one gene, two more terms come into play:
- Genetic heterogeneity. Mutations in more than one gene can cause the same phenotype. Famous case: parents were found guilty of child abuse because their kids had broken bones and the parents didn't have mutations in the one gene then known to cause osteogenesis imperfecta. They had mutations in a different gene that also breaks bones – several are now known.
- Epistasis. One gene affects another. Spinal muscular atrophy (SMA1) is like severe, rapid ALS in a child. The abnormal protein shortens axons, hastening motor neuron death. In one family, one child died very young, but another lived several years longer. The more fortunate sibling had a variant in a different gene, plastin 3, which extends axons.
Similarly, siblings who both inherit Huntington's disease (last week's post) may get sick at different ages based on the rate of division of stem cells in the brain, the luckier individual making up for the cell death of HD faster than the other. In fact, if the course slows enough, the person may die of something else and never know she or he had HD, unless there was a genetic test. That looks like a healthy person with a devastating mutation. A similar scenario is true for Alzheimer disease. Mutations in apoE4 increase risk, while mutations in the amyloid precursor protein gene lower the risk.
SMA1, HD, and Alzheimer disease are devastating brain diseases. The idea that a person could have a single-gene test that finds a mutation, but the associated disease could be tempered by a variant of a different gene that isn't tested for, scares me.
Not recognizing a genetic disease
Another explanation for how a healthy person can have a genetic disease is that people, and sometimes their physicians, aren't thinking about genetics, especially when a symptom is common. Leslie Biesecker, corresponding author of the new paper, offers an example: "A couple of the participants with LDLR (low density lipoprotein receptor) mutations thought they just had garden variety high cholesterol, when in fact they had familial hypercholesterolemia."
The study also found people with heart defects, immune deficiencies, blood disorders, blistering feet, or stubby fingers who had no idea that they or their family members had an inherited disease. Further investigation found biochemical evidence, symptoms, or telling characteristics.
One family with lipodystrophy due to mutation in the PPARG gene thought they all just had prominent musculature which was unrelated to their diabetes or abnormal lipid profiles. Another had hair follicle tumors caused by a mutation in the FLCN gene. That was important to know because it elevates risk of kidney cancer. And speaking of cancer, 19 of the participants had cancer susceptibility mutations well known for their incomplete penetrance, such as the BRCA genes. Only 6 of the 19 had family histories of the associated cancers.
I can think of lots of other examples of unrecognized genetic disease:
- Chronic sinusitis may be cystic fibrosis.
- Bleeding easily and for a long time may signal a clotting factor deficiency, and the study indeed found 5 cases.
- Highly bendable body parts may be due to Ehlers-Danlos syndrome.
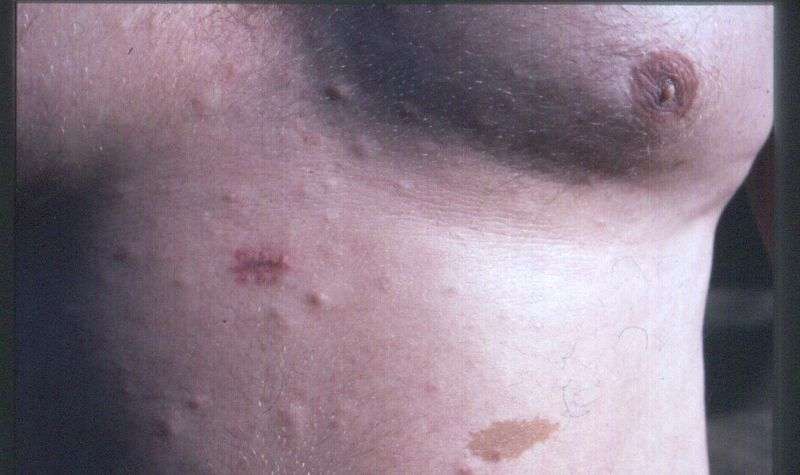
- A person with a few café-au-lait spots may have neurofibromatosis.
- I don't sweat much, and neither did my mother nor her mother. I've wondered if I have hypohidrotic ectodermal dysplasia, but only the one symptom.
Sometimes, the genetic evidence is just a mystery. In the study, for example, four people should have had glaucoma and two should have cataracts, according to their genes—but didn't.
Practically speaking, people who don't receive regular health care, or if they do see a health care provider for 5 minutes, would not have an opportunity to assemble these and more subtle clinical puzzle pieces. And that's the value of the study—exome sequencing can clearly change some peoples' risks of a particular condition from one in thousands to about one in two, depending upon the detected mutation and its penetrance.
Incidental findings on a grand genomic scale
Earlier exome and genome sequencing experiences predicted that we'd find disease-causing genotypes among healthy people. Consider "incidental findings" (aka secondary or unsolicited) – when a medical work-up looks for one thing and finds another. Three early cases from exome screens for adults with atherosclerosis or children with developmental delay come to mind:
- A family whose members thought they had writer's cramp actually had a neurological disease, myoclonus dystonia.
- A child had undetected Marfan syndrome. His aorta was at risk of bursting without treatment.
- A man who had been deaf from birth never realized it – he thought all people read lips.
Trolling exomes or genomes for mutations is a little like finding humor in a Stephen King horror novel. Look for one thing, find another. It happens, more often than we thought.
We must decipher gene-gene interactions
The new findings change the estimate of 0.02% of the US population having a genetic condition to as high as 3%, about the size of the population of New Jersey, the researchers point out. Now we need to find out why and how some genotypes cause symptoms in one individual but not in another.
To do that, we must leap beyond sequencing, to decipher all the ways that all the genes and their many variants can interact. Gene-gene interactions may explain why the boy with sickle cell disease is hospitalized often, in excruciating pain, while his sister who also has the disease has a much easier life: they've inherited different combinations of variants of other genes that impact the functioning of the mutant beta globin gene that they share.
Unraveling gene interactions can even reveal new drug targets. For example, the study found two men who had mutations known to cause Duchenne muscular dystrophy—but they didn't have the disease, which surely would have been obvious in boyhood. What second gene, or circumstance or environmental factor, protects them?
Figuring out all the interactions that underlie gene expression is a challenge that dwarfs that of sequencing the first human genome, and the effort is underway. Now that's precision medicine.
More information: "Individualized Iterative Phenotyping for Genome-wide Analysis of Loss-of-Function Mutations." DOI: dx.doi.org/10.1016/j.ajhg.2015.04.013