Quantitative assessment of dynamic deformability and adhesion of red blood cells possible
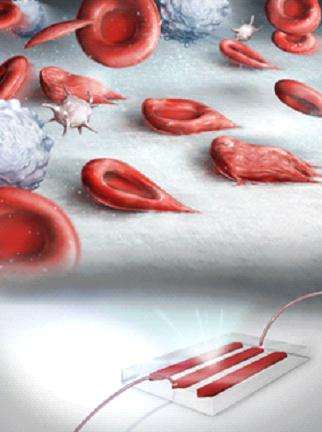
A team of researchers from the Case Western Reserve University (CWRU) in Cleveland, OH have developed a versatile microfluidic platform integrated with a cell dimensioning algorithm for quantitative assessment of dynamic deformability and adhesion of RBCs in controlled microphysiological flow. Accurate measurement of RBC deformability and adhesion, which are the two key biophysical factors of vaso-occlusion in SCD, holds great potential as a marker for evaluation of disease progression, gaining insight into disease pathophysiology, and development of novel therapeutics. Although various approaches have been utilized for measurement of deformability and adhesion of RBCs, such as atomic force microscopy and optical tweezers, none of these methods could be conducted at physiological conditions using whole blood in a clinically relevant way. The developed microfluidic system can probe deformation characteristics of RBCs at the single cell level, as well as reflecting microvasculature adhesion response in whole SCD patient blood samples.
"Microfluidic techniques allow incorporation of physiological flow conditions, as well as biologically relevant adhesion surfaces in a closed setting, which better mimic the natural physiological environment of the RBCs in blood flow. The microfluidic system developed here has the potential to be used in a high-throughput manner with an integrated automated image processing algorithm for measurement of RBC deformability and adhesion in patients' blood." says Professor Umut Gurkan, Ph.D., of the Case Western Reserve University and Principal Investigator on the paper.
Red blood cells (RBCs) undergo dynamic reversible deformations in blood circulation and respond to fluid shear stresses rapidly with time constants in the range of 100 milliseconds. However, RBCs lose their ability to deform dynamically with maladies such as diabetes, malaria infection, hereditary spherocytosis, and various mutations affecting globin genes, such as the sickle cell disease (SCD). SCD is the first recog-nized molecular disease, which was identified as a hemoglobin disorder more than sixty years ago. In the roots of the disease is a point mutation in the 6th chain of the hemoglobin gene, which results in abnormal polymerization of hemoglobin molecules inside the RBCs. Formation of polymerized hemoglobin fibers disrupts cell morphology, decreases RBC deformability (increase in stiffness), and changes membrane adhesive properties. Abnormal adhesion and decreased deformability of RBCs are the main causes of blood vessel occlusion (vaso-occlusion) in SCD. Vaso-occlusion is the hallmark of the disease and it has been associated with severe pain, crises, wide-spread organ damage, and early mortality.
Molecular basis of the SCD have been investigated extensively. However, there are limited number of studies focusing on the biophysical factors in tandem, such as the deformability and the adhesion of RBCs, which are highly dynamic phenomena. Even though RBC deformability has been associated with vaso-occlusion in SCD, we have limited knowledge on dynamic deformation characteristics of RBCs adhered to endothelium associated proteins in microphysiological fluid flow conditions. While various approaches have been utilized for the measurement of RBC deformability, including optical tweezers, micropipette aspiration, atomic force microscopy (AFM), and microfluidics. Even though optical tweezers, micropipette aspiration, and AFM analyses have enabled sensitive and controlled measurement of RBC mechanical properties, these methods are typically performed in open environments without fluid flow.
The developed microfluidic platform by the CWRU researchers can probe dynamic deformation behavior of adhered RBCs under physiological flow conditions at the single cell level. To assess dynamic deformability of RBCs, the researchers introduced a new parameter: dynamic deformability index (DDI), which they defined as the time dependent change of the cell's aspect ratio. "Using this microfluidic system, we ana-lyzed dynamic deformability and adhesion of sickle RBCs at physiological and above physiological flow shear stresses. We report for the first time on the subpopulations of RBCs in terms of dynamic deformation characteristics in SCD: deformable and non-deformable RBCs. Furthermore, we analyzed adhesion of non-deformable RBCs, in comparison to deformable RBCs, quantitatively at physiological and above physiological flow shear stresses in blood samples obtained from SCD patients. We observed significantly greater number of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well-above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events." says Yunus Alapan, Ph.D. candidate, the lead author on this paper.
A unified investigation of adhesion and deformability properties of RBCs may have significant implications for understanding vaso-occlusion events and for phenotyping disease pathophysiology. Studying dynamic deformation of cells may have implications in other multi-system diseases such as β-thalassemia, diabetes mellitus, hereditary spherocytosis, polycythemia vera, and malaria. The team from CWRU is now working to further characterize deformability and adhesion of RBCs in greater number of SCD patients to analyze their associations with clinical phenotypes and complications. This adaptable technology may give important biophysical insights into disease pathophysiology when widely applied in SCD. Further-more, the developed microfluidic platform has the potential to be used as an in vitro assay for monitoring disease activity, at baseline and during clinical flux after treatment, during painful episodes, and in association with long-term complications.
More information: Y. Alapan et al. Dynamic deformability of sickle red blood cells in microphysiological flow, TECHNOLOGY (2016). DOI: 10.1142/S2339547816400045