Researchers block common colon cancer tumor type in mice
A new scientific study has identified why colorectal cancer cells depend on a specific nutrient, and a way to starve them of it. Over one million men and women are living with colorectal cancer in the United States. The National Cancer Institute estimates 4.5% of all men and women will be diagnosed with the cancer during their lifetime, making it the third most common non-skin cancer.
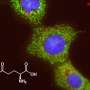
In the study published online in Nature Communications, researchers showed how certain colorectal cancer cells reprogram their metabolism using glutamine, a non-essential amino acid. Many cancer cells rely on glutamine to survive. How they become so dependent on the molecule is hotly debated in the field.
Researchers studied a subset of colorectal cancer cells containing a genetic mutation called PIK3CA. This mutation is located in a gene critical for cell division and movement, and is found in approximately one third of all colorectal cancers. The mutation is also the most commonly identified genetic mutation across all cancers, making the results of the study universally appealing.
Researchers were interested in determining whether or not the common PIK3CA mutation contributes to changes in cancer cell metabolism, such as how nutrients like glutamine are processed. Normally, glutamine is broken down by cancer cells into several other molecules with the help of specific enzymes. This complicated system helps produce adenosine triphosphate, the energy currency of all cells, and other molecules critical for colorectal cancer cell growth.
The researchers found that colorectal cells with the PIK3CA mutation broke down significantly more glutamine than cells without the mutation. The researchers identified several enzymes involved in the process that are more active in the mutant cancer cells than in other cell types, explaining the increased need for glutamine. These enzymes become overactive in the mutant cancer cells due to a cascade of signals led by the protein encoded by mutant PIK3CA gene. This finding represents a novel and important link between the common PIK3CA mutation and altered glutamine metabolism in cancer cells.
Zhenghe John Wang, PhD, professor of genetics and genome sciences and co-leader of the Cancer Genetics Program at Case Western Reserve University School of Medicine helped lead the study. "In layman's terms, we discovered that colon cancers with PIK3CA oncogenic mutations are addicted to glutamine, a particular nutrient for cancer cells. We also demonstrated that these cancers can be starved to death by depriving glutamine with drugs."
When the researchers lowered the amount of glutamine available to mutant cancer cells growing in laboratory dishes, the cancer cells died. This discovery led the team to investigate the effects of blocking glutamine availability in mice with colorectal cancer tumors containing the common PIK3CA mutation. Wang and colleagues found that exposing these mice to a compound that blocks glutamine metabolism consistently suppressed tumor growth. They did not observe the same effect on tumors without the mutation. Together, these results provide a promising new therapeutic avenue to suppress growth of colorectal tumors with the PIK3CA mutation. The researchers have filed a patent application based on the unique mechanism of tumor suppression they have identified and the work is available for licensing.
"This study provides the basis for a colon cancer treatment clinical trial that will be started in the summer at the University Hospitals Seidman Cancer Center," according to Neal Meropol, MD, Dr. Lester E. Coleman, Jr. Professor of Cancer Research and Therapeutics, chief of the division of hematology and oncology, and principal investigator for the trial. The phase I/II study will test the effects of a glutamine metabolism inhibitor in patients with advanced colorectal tumors.
More information: Yujun Hao et al, Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer, Nature Communications (2016). DOI: 10.1038/ncomms11971