Structural changes in taste receptors in the first stage of taste perception discovered
For the first time Japanese researchers discovered that the extracellular domain of taste receptor proteins undergoes a change in structure by binding together taste substances. This structure change is thought to transmit the extracellular binding of taste substances to taste cells. The findings will contribute to our basic understanding of taste mechanisms and the development of a new taste evaluation system that makes use of the screening and detection of taste receptor structure changes.
Our sense of taste enables us to recognize the chemical substances in food and form judgments as to whether these include nutrients that are necessary for our survival or poisonous substances that would cause us harm. For each of the five basic tastes of sweet, umami, salty, sour and bitter, we possess respective sensor protein taste receptors. In the fields of molecular biology and biochemistry it has become possible to produce a variety of biological proteins using cells in experimental settings. However, producing a greater quantity of taste receptors and maintaining their proper structure and function in lab settings has so far been unsuccessful. This has prevented their precise analysis resulting in a lack of understanding of the detailed processes of taste substance binding and related processes.
A group of researchers led by Atsuko Yamashita, Professor at the Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Eriko Nango, Researcher at the RIKEN SPring-8 Center, Yuji Ashikawa, at the time Researcher at the RIKEN SPring-8 Center, Saori Maki, Researcher at the RIKEN SPring-8 Center, Shuji Akiyama, Professor at Institute for Molecular Science, National Institute of Natural Sciences, Yuko Kusakabe, Unit Leader at the Food Research Institute, National Agriculture and Food Research Organization, and Susumu Uchiyama, Assistant Professor at the Graduate School of Engineering, Osaka University now made the discovery that the extracellular domain of taste receptor proteins changes when taste substances bind together.
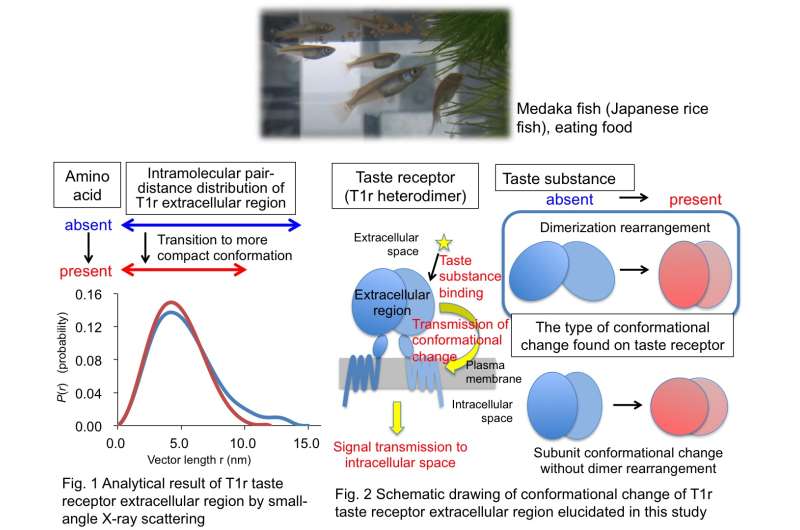
In their study, the researchers were able to produce extracellular domains of T1r2 and T1r3 heterodimer taste receptors of Medaka fish using cultured insect cells. The so-called T1r (Taste receptor type 1) family consists of proteins that serve as taste receptors recognizing sweet tastes and glutamine acid (umami taste). These receptors are located in the extracellular domain, the transmembrane, which is embedded in the cell membrane, and the intracellular domain. They achieve their function as sweet and umami receptors by forming heterodimers (a state in which two different types of proteins are grouped together). Since two thirds of the T1r family receptors protrude from taste cells and therefore are exposed within the mouth, this extracellular domain is considered to be the main binding region for taste substances. In other words, taste perception starts in this extracellular domain where taste substances bind together. In the study, the researchers clarified that the extracellular domain structure of taste receptor protein changes into a compact state as taste substances bind together. They also made the finding that this structure change is based on the reciprocal influence of the protein molecules that make up heterodimers. Based on their study findings, the researchers inferred that information on taste substance binding taking place outside of the cell is transmitted to the remaining one third of receptors located in the transmembrane and the intracellular domain via this structure change.
The research results represent a major step towards conducting precise analyses using purified protein samples produced in the laboratory. In addition, a system evaluating the binding of taste substances by detecting the structure change of taste receptors was created (patent application 2013-246300). If the production of human taste receptors becomes possible, this can lead to the development of an evaluation system for taste substances capable of discerning what humans perceive as palatable.
More information: Eriko Nango et al. Taste substance binding elicits conformational change of taste receptor T1r heterodimer extracellular domains, Scientific Reports (2016). DOI: 10.1038/srep25745