Fat cells that amplify nerve signals in response to cold also affect blood sugar metabolism

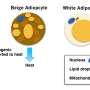
When exposed to cold, clusters of cells within the body's white fat become beige – a color change that reflects the creation of more energy-producing mitochondria, cellular components that enable cells to burn calories and give off heat. But since white fat cells have very few nerves, how do beige fat cells get the message that it's cold outside?
In research that has implications for diabetes and other metabolic diseases, an international study based at UT Southwestern Medical Center found that the protein connexin 43 (Cx43) forms cell-to-cell communication channels on the surface of emerging beige fat cells that amplify the signals from those few nerve fibers. The channels act like conduits that speed signals across the gaps between clusters of cells – similar to the way a group email reaches several people at once.
The study, recently published in Cell Metabolism, also found that beige fat, unlike the better-known white and brown fat, has interesting anti-diabetic effects on blood sugar metabolism that seem independent of temperature regulation. Impaired glucose metabolism is a hallmark of diabetes.
"The data here show how white fat cells can make maximal use of their limited number of nerves to allow a single nerve fiber to spread the 'message' about cold temperatures amongst the connected cells," explained Dr. Philipp Scherer, lead author of the study and Director of UT Southwestern's Touchstone Center for Diabetes Research.
"To my knowledge, this is the first time that any fat's thermal regulatory (warming) and metabolic effects on blood sugar have been observed to work independently. Our findings suggest that activating Cx43 may cause the formation of more beige fat and thus increase the anti-diabetic effects seen in this study," Dr. Scherer added.
Fat, once considered merely a storage area for excess calories, is now appreciated as a dynamic tissue that comes in several forms with different functions that are still being identified. White fat is used mainly for energy storage. Brown fat, the classic heat-generating fat, helps regulate body temperature, especially in newborns. Some white fat cells are capable of transforming into a third kind of fat, beige. Both brown and beige fat get their color from increased mitochondria that are added in response to cold and other environmental stimuli, he said.
To study the metabolic effects of beige fat, the researchers compared mice with Cx43 that are able to make beige fat normally to mice unable to make Cx43, meaning their white fat seldom got the message to change to beige in response to cold. After three weeks in cold temperatures, the mice were returned to normal temperatures and analyzed for glucose (blood sugar) metabolism.
The mice that produced Cx43 showed greater improvement in glucose metabolism, Dr. Scherer said. Yet, both groups of animals were still able to regulate body temperature, apparently through their brown fat stores, he said.
"This study reaches two conclusions: First, Cx43 is necessary for the propagation of nerve signals that lead to beiging of white fat tissue. Second, beige fat may be more interesting from an anti-diabetic, metabolic standpoint – a finding with significant clinical relevance – than from a body temperature, warming standpoint," explained Dr. Scherer, who holds the Gifford O. Touchstone, Jr. and Randolph G. Touchstone Distinguished Chair in Diabetes Research.
Study co-authors from the Touchstone Diabetes Center included lead author Dr. Yi Zhu, a postdoctoral fellow and employee of Eli Lilly and Co.; former graduate student Dr. Caroline Tao; postdoctoral researchers Dr. Mengle Shao and Dr. Shangang Zhao; Dr. Olga Gupta, Assistant Professor of Pediatrics and Internal Medicine and a Dedman Family Scholar in Clinical Care; and Dr. William Holland and Dr. Rana Gupta, both Assistant Professors of Internal Medicine. Additional UTSW contributors were Joshua Johnson, a student in UT Southwestern's Medical Scientist Training Program; Dr. Tiemin Liu, Instructor of Internal Medicine; Dr. Joel Elmquist, Chief of the Division of Hypothalamic Research, Professor of Internal Medicine, Pharmacology and Psychiatry, and holder of the Maclin Family Distinguished Professorship in Medical Science, in Honor of Dr. Roy A. Brinkley, and the Carl H. Westcott Distinguished Chair in Medical Research; and Dr. Kevin Williams, Assistant Professor Internal Medicine in the Division of Hypothalamic Research.
Researchers at the Chinese Academy of Medical Sciences and Peking Union Medical College; The Ohio State University; Xi'an Jiaotong University; the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (NIH); Albert Einstein College of Medicine; and Joslin Diabetes Center also contributed.