Study of malaria rapid diagnostic tests in Uganda assesses scalability, identifies supply chain challenges
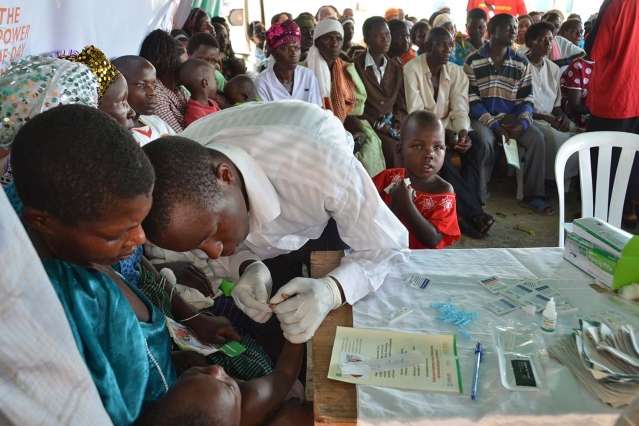
The World Health Organization estimates that nearly half of the world's population is at risk for malaria, a life-threatening, but ultimately curable and preventable disease spread by mosquitos.
Malaria rapid diagnostic tests are an important part of the fight against the disease, but according to new research from MIT, supply chain challenges keep these tests from making it onto clinic shelves, putting patients at risk of misdiagnosis, which over time can lead to antibiotic resistance.
MIT researchers have just released a new report evaluating these malaria rapid diagnostic supply chains in Uganda, where misdiagnosis is common and where many patients turn to the private sector—local pharmacies, clinics, and drug shops—for malaria treatment and care.
The report, "Evaluating Business Criteria for Scaling Stock of Malaria Rapid Diagnostics," details the study design and findings of the latest experimental evaluation implemented by the Comprehensive Initiative on Technology Evaluation (CITE), a program supported by the U.S. Agency for International Development (USAID) and led by a multidisciplinary team of faculty, staff, and students at MIT.
Launched at MIT in 2012, CITE is a pioneering program dedicated to developing methods for product evaluation in global development. CITE researchers evaluate products from three perspectives, including suitability, or how well a product performs its purpose; scalability, or how well the product's supply chain effectively reaches consumers; and sustainability, or how well the product is used correctly, consistently, and continuously by users over time.
Service, not a product
To conduct this evaluation, MIT researchers worked closely with the Malaria Consortium, a nonprofit organization that specializes in the prevention, control, and treatment of malaria worldwide. In 2014, the Malaria Consortium established a pilot program to increase availability of malaria rapid diagnostics in Uganda's private sector, where they had observed stocks were limited or non-existent.
"MIT's research focused on the private sector's willingness to stock malaria rapid diagnostics," said CITE scalability lead Jarrod Goentzel. "The availability of diagnostic technologies at the local pharmacy where sick people initially seek care is critical in making sure they receive the right treatment."
To better understand the challenge, CITE conducted interviews and focus groups with key supply chain actors involved in the Malaria Consortium's pilot study: both first-line buyers, all three distributors, and 28 retailers.
Through a literature review, interviews, and these focus groups, CITE identified 17 criteria that supply chain actors considered when making decisions about whether or not to stock malaria rapid diagnostic tests. Then, researchers used a management science technique called "multi-criteria decision analysis" to better understand which of the 17 criteria were most important to individual actors' decision-making.
"One of the primary insights was that retailers were more concerned about their time to make the diagnosis than the cost of the technology," Goentzel explains. "Their satisfaction with stocking the technology dropped significantly when the 'time for sale' went longer than 10 minutes."
The retailers were involved in four key steps for every malaria rapid diagnostic test they sold. First, the consultative sales transaction, then administration of the test, followed by a waiting period, and finally, interpretation of results.
"This process takes time away from their other business, making them less inclined to stock the product," Goentzel says. "We recommend that going forward, malaria diagnostics should be framed as a service to be provided rather than a product to be sold."
Retailers interviewed for the study also valued training on how to administer malaria diagnostics, a fundamental requirement that enables them to offer these diagnostic services in the first place.
Business model alignment across the supply chain
While retailers emphasized the importance of training and time of sale, distributors in the study identified expiration date and sales volume as the most important criteria in their decision to stock malaria diagnostics. The shelf life for malaria rapid diagnostics is measured in months, not years, and while retailers are able to place small orders from distributors to avoid expiration risk, distributors are often left with expired product.
"In this malaria rapid diagnostic supply chain, distributors were bearing most of the financial risk," Goentzel explains. "Additionally, they were asked to provide training to the retailers, which is a major investment of their time."
These criteria were reflected in the CITE evaluation of four potential business models for selling malaria rapid diagnostic tests, including:
- selling World Health Organization (WHO)-approved products with no bundled services;
- selling WHO-approved products using Malaria Consortium's bundled services;
- selling products not approved by the WHO; and
- not stocking the products at all.
First-line buyers and retailers preferred the bundled services option because of the high value they place on training. On the other hand, distributors preferred selling malaria rapid diagnostics outside of the bundled services due to issues highlighted earlier.
The research team recommend devising different business models to share risk and balance responsibility across all actors in the supply chain. "Modifying aspects of the bundled service model might make this option more attractive to the distributors going forward, which would drive sales volumes across the supply chain," Goentzel added.
More information: Malaria Rapid Diagnostic Test Evaluation: cite.mit.edu/reports/malaria-r … tic-test-evaluation#
This story is republished courtesy of MIT News (web.mit.edu/newsoffice/), a popular site that covers news about MIT research, innovation and teaching.