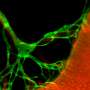
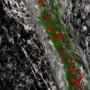
Fat fuels the road to cancer cell spread
Cancer cells spread to other sites in the body through promoting the growth of new 'roads' to travel on. In a study to be published on December 26th in the top scientific journal, Nature, an international and multidisciplinary team of researchers, led by Prof. Dr. Peter Carmeliet (VIB-KU Leuven), discovered how a shift to increased fat utilization is required for the development and growth of these 'roads', termed lymphatic vessels - a special kind of blood vessels. This discovery paves the way towards developing therapeutics to limit lymphatic vessel growth in cancer by targeting fat utilization.
The spread of cancer, termed metastasis, is one of the most important and life-threatening complications of cancer today. Current chemotherapy and radiotherapy can effectively treat many cancers, however, the spread of cancer cells to multiple sites within the body results in the majority of deaths associated with cancer. In order for cancer cells to spread, they must find a pre-existing 'road', or build a new 'road' to travel on. Lymphatic vessels, a specialized kind of vessels transporting fluid rather than blood, are a primary route of cancer cell spread, and the formation of new lymphatic vessels, termed lymphangiogenesis, is a poorly understood process, which currently lacks clinically approved drugs to prevent their growth during disease.
Fat fuels lymphatics
Expanding upon recent work in the laboratory published in top journals such as Cell and Nature, a team consisting of Drs. Brian Wong, Xingwu Wang and Annalisa Zecchin, guided by Prof. Carmeliet sought to investigate the nutrient utilization (metabolism) of lymphatic vessels. The study began with a simple observation: lymphatics use more fat (fatty acids) compared to blood vessels. This is the first description of the nutrient utilization of lymphatic vessels. Using drugs to prevent fat utilization by lymphatics prevented lymphatic growth, an important step in translating this finding to the cancer setting and inhibition of metastasis.
What cells eat determines what they become
To understand why these cells are so reliant on fat, the researchers investigated how lymphatics develop. Lymphatics 'transform' from blood vessels during embryonic development, and this study shows that the signals that transform blood vessels to lymphatics also change their 'taste' to prefer eating fat. The novelty of this discovery is that this 'transformation' relies on an increase of fat utilization. In this process, the fat is used to generate molecules which can modify important factors that regulate the expression of the genetic code, termed epigenetic changes, which can ensure the function of lymphatics. The hard-wiring of the genetic code (DNA) itself is not altered by fat, but the utilization of this code that defines the lymphatic gene signature is modulated. A key translational aspect to this finding was the proof that resupplying another (fat) nutrient source could restore the growth and function of lymphatics.
Dr. Brian Wong (VIB-KU Leuven): "Our study shows that the usage of fat by lymphatics is programmed in their development, and required for their growth and function. We have demonstrated by enhancing or preventing the usage of fat (or fat byproducts), we can control the growth of lymphatics."
The next steps to preventing cancer cell spread and treating cancer patients
The immediate next steps of this research are clear and two-fold. On one hand, inhibitors of fat usage will be tested on a large scale for their ability to reduce metastasis in different types of cancer. On the other hand, we will test whether dietary fat supplements (for instance in the form of ketone bodies, used by athletes) can heal faulty lymphatics, a major complication in cancer patients undergoing surgical cancer removal, which leads to the debilitating swelling and dysfunction of the arms and legs, termed lymphedema, for which no drug is available.
Prof. Peter Carmeliet (VIB-KU Leuven): "Our immediate next studies are focusing on further translating these findings to the cancer setting. Previously, we could not develop drugs to target the growth of lymphatic vessels because we did not understand how they develop and function. Our work demonstrates the importance of their reliance on fat, and provides essential steps towards developing effective drugs to prevent excessive lymphatic growth in cancer, but also to treat incapacitating complications of lymphedema."
More information: Brian W. Wong et al, The role of fatty acid β-oxidation in lymphangiogenesis, Nature (2016). DOI: 10.1038/nature21028