Researchers pioneer HIV research with 'kick and kill'

As his title implies, for David Margolis, MD, director of the UNC HIV Cure Center, treating the virus and its symptoms simply isn't enough.
Instead, Margolis and his team at the UNC Cure Center and Collaboratory of AIDS Researchers for Eradication (CARE), are working with the end goal in sight: a cure.
"We're trying to develop a new medical therapy – something that has never been done – the eradication of a viral infection that is integrated into the host's genome," Margolis said. "This is essentially like curing a cancer."
Margolis started this work at a time when many doctors and scientists didn't even want to use the word "cure" when discussing treatments for HIV. They viewed it as impossible and irresponsible to give people living with HIV false hope for a cure. Margolis forged ahead anyway, even though he knew how difficult it would be to rid the body of HIV.
When a person is infected with the virus, it becomes part of some cells within that individual's immune cells, Margolis, professor of medicine, microbiology and immunology at UNC School of Medicine, said. When given certain therapies and medications, an HIV-positive person can display signs that the virus is gone. But it's not. Small amounts of HIV become latent or dormant – essentially hiding within the depths of that person's own organs and systems.
"We can stop HIV from growing with current drugs and restore health, but people are left with a chronic infection and require chronic care and chronic therapy," Margolis said. "One approach is to change the human immune system in some way so that it can control HIV for the lifespan of the person. There are some groups that are working on that. We think it's a worthy goal, but it's very difficult, and will still leave someone with a chronic, low-level infection.
"The approach our group has pursued for quite some time is to eradicate the infection itself," Margolis said. "We've had growing support from the National Institutes of Health for the last 10 years, and the CARE project has now been renewed for another five years. Our 'kick and kill' approach is the foundation of our partnership with GlaxoSmithKline, this unique public-private research partnership here at UNC is to seek a true cure."
The partnership between UNC and GlaxoSmithKline (GSK), a global, research-based pharmaceutical developer, officially kicked off in May 2015. GSK has agreed to invest $4 million per year for five years to fund the research plan and the team. The university provides the lab space on the medical campus.
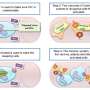
"We have a strategy and it is to disrupt latency and make those latent cells visible in some way to the immune system, and then arm the immune system in a way that it allows clearance of those infected cells. But that's not something that can be done in an instant or that you can take a pill and it will happen. It's a therapy, like chemotherapy, that's going to have to happen over time," Margolis explained.
But where does the virus hide?
"It goes everywhere," Margolis said. "It's really not a question of whether it's going to some small place to hide out; it's really more a question of can you get your latency-reversing therapy and your clearance response to the place where those viruses are hiding?"
The "kick"
The first drug that has proven to have potential in disrupting the virus' latency was Vorinostat, which was initially used to treat cancer. Margolis saw its potential and began testing it to target the virus and "kick" it out of hiding.
"Not only did Vorinostat prove to be able to reverse viral latency, but it also became evident that it could potentially make the latent virus visible for clearance – meaning attacking the virus with either a vaccine or via immunotherapy" Margolis said.
But finding the virus and flushing it out is only the beginning.
"Just because you find a drug that can reverse latency, it doesn't mean that you know how to use it," Margolis said.
That's where Nancie Archin comes in.
In 2012, Archin, assistant professor of medicine, UNC School of Medicine Division of Infectious Diseases, published what Margolis described as "a seminal paper" on Vorinostat in the journal Nature. Archin's most recent findings in her work with Vorinostat have to do with identifying the proper dosing. She has submitted the paper for publication, and she's confident she has pinpointed the proper dosing of the drug to effectively "kick" the virus out of latency.
"We're at the beginning of trying to figure out how this could work – how to do this," Margolis said. "So every step that we do is a new step and has to be done from scratch and figured out from scratch. And that has to be very carefully tested and figured out – both to figure out how you can disrupt latency serially over time until every single latent infection has been exposed to the immune system, and how you can get that virus cleared over time. It's a complicated, combination therapy that has to be worked out step-by-step."
Margolis expects to make significant advances in this method in the coming years.

"But we don't expect to cure anyone in the next couple of years. Since 2012, we've been trying to figure out how to use Vorinostat in a safe and effective way that disrupts latency but doesn't screw up the immune system's response, because the two kinds of approaches have to work together, and that's the really exhaustive work that Dr. Archin has led."
Archin found that when Vorinostat was administered too frequently, it became less effective and even counter-productive.
"We had to take a step back and try to determine what the correct dosing interval of Vorinostat should be. Based on our preliminary data, we were able to show that Vorinostat, if given every single day, could be counter-productive," Archin said. "What we did in the lab was try to model what a dosing schedule of Vorinostat should be."
When doses were administered between 48 and 72 hours apart, the effectiveness seemed to return, she said.
"Patients who were enrolled in the dosing schedule of the Vorinostat study were given a single dose, then they came back and received the second dose in that 48- to 72-hour window," Archin said. "Subsequently, we studied additional patients, on all of whom the Vorinostat dosing worked. Now that we have the proper dosing schedule, our next studies will combine the Vorinostat to 'kick' the virus with either a vaccine or immunotherapy to 'kill it.'"
The "kill"
The "kill" studies are underway at UNC. Margolis' lab, as well as Cynthia Gay, MD, MPH, associate professor of medicine, have teamed up with a Durham pharmaceutical developer, Argos, which has developed a vaccine to use in the "kill" portion.
"We've been trying to run things in parallel tracks so that we can move things forward as quickly as possible," Margolis said. "We have to test very carefully how each component of the kick and kill strategy will work together.
"While Dr. Archin was leading the study to understand how to use Vorinostat, Dr. Gay and other scientists at UNC were leading the trial study using the Argos vaccine on patients who were infected but suppressed on therapy. In the study, they would give patients doses of the Argos vaccine to see not only whether an immune response to HIV was induced by the vaccine, but importantly, how long it lasted because we need to know the immune response is around when we're flushing the virus out," Margolis said.
The "kick and kill" trials are exhaustive, but that comes with the territory when a lab is working to eradicate a virus that affects more than 35 million people across the world.
"This is challenging, time-consuming, even painstaking work. That said, we're working to find curative therapies for HIV patients, and we're working as hard on this as we can," Archin said.
More information: N. M. Archin et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy, Nature (2012). DOI: 10.1038/nature11286