Clinical trial reveals a safer long term treatment for blistering skin disease
A clinical trial into the treatment of the severe blistering skin condition 'bullous pemphigoid' has found that starting treatment with an oral antibiotic is an effective and safer alternative to the current standard treatment of oral steroids which can have harmful long-term side effects.
The study was led by skin experts at The University of Nottingham's Centre of Evidence Based Dermatology. The results of the NIHR-funded BLISTER trial are published in The Lancet.
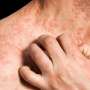
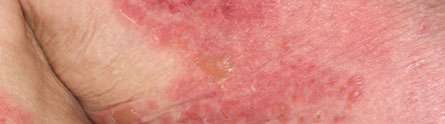
Bullous pemphigoid (BP) is an auto-immune disease which means that the immune system mistakenly makes antibodies against parts of the patient's skin. This causes an itchy rash and blisters anywhere on the body,which can become infected if they burst.
Professor of Dermatoepidemiology at Nottingham, Hywel Williams said: "Bullous pemphigoid is a miserable condition which can severely affect the quality of life of hundreds of elderly people in the UK every year."
80 year old Brenda Harrison from Hull was one of the volunteer patients who took part in the BLISTER trial. She said: "I'm currently recovering from my fourth attack of bullous pemphigoid in five years and it is a very unpleasant condition to cope with. I am on steroids which make you gain weight and I have to dress to cover the blisters and scabs which are unsightly. If it wasn't for my husband, I would not be able to cope with applying the cream twice a day as I can't reach the areas on my back which are affected. I think the Blister trial is much-needed and the results will help more people like me."
The research team at Nottingham set out to find out whether starting treatment with a commonly-used antibiotic 'doxycycline' would be an effective and safer alternative to the current standard treatment of the oral steroid 'prednisolone'. These steroid tablets work well but can cause long term harmful side effects including diabetes, high blood pressure, fractures, infection and eye problems.
54 UK hospitals and 7 German hospitals took part in the randomised, controlled study. A total of 253 patients were recruited of which 132 were started off on doxycycline and 121 started off on prednisolone. After 6 weeks, patients could switch treatments or increase the dose of prednisolone as needed, as would typically occur in practice.
The trial showed that the steroid prednisolone was slightly more effective in controlling symptoms of the disease but it carried a higher risk of severe, life threateneing and fatal side effects. For those patients given doxycycline, 74% had fewer than 3 blisters after six weeks compared to 91% or patients on prednislone. But only 18% of patients on the antibiotic severe suffered adverse effects after 1 year compared to 36% of those on the steroid treatment.
Professor Williams said: "Although the safest form of treatment for pemphigoid are steroid creams applied in large quantities to the skin for long periods, such treatment is not practical for everyone, and tablet treatments are needed. Steroid tablets are good at controlling blisters, but they have serious long term side effects in the elderly. Starting off treatment with doxycline offers reasonable short-term blister control with much better long-term safety when compared with oral steroids."
More information: Hywel C Williams et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial, The Lancet (2017). DOI: 10.1016/S0140-6736(17)30560-3