Unusual metabolic activity illuminates embryonic development, cancer
Move over, cell signaling. Scientists seeking to understand embryonic development need to make room for metabolism, according to a new study, led by Harvard Medical School developmental biologist Olivier Pourquié, that also has implications for regenerative medicine and cancer research.
For decades, scientists crowned cell signaling—that is, communication within and among cells—the king of embryonic development, decreeing which cells go where and what tissues they ultimately become. Energy metabolism, the thinking went, hummed along in the background, uniform across every cell.
The new study, published in Developmental Cell, reveals that a particular type of metabolism—one that is also a hallmark of cancer cells—actually varies in different parts of an embryo, surging in areas where the organism is busy growing longer.
Far from playing a passive role, the research reveals, this metabolic activity helps integrate cell signaling to control embryo elongation and cell fate.
A companion paper by a separate research team in the same journal reiterates the findings.
The work calls attention to an overlooked player in embryonic development, according to Pourquié, who is a professor of genetics at HMS, the Frank Burr Mallory Professor of Pathology at Brigham and Women's Hospital and a member of the Harvard Stem Cell Institute.
"So far, the field has been concerned with genes and proteins; now we also have to include metabolites, because we show they control the expression of genes and signaling," he said.
"Developmental biologists have learned a lot by playing with genes and proteins, but we were a bit stuck because we were missing some factors," Pourquié added. "I think metabolism will explain a lot about the phenomena we've been looking at."
The work could lead to a better understanding of cancer physiology, the authors say. It also opens the door for researchers to improve their understanding of the metabolic needs of stem cells so they can better engineer tissues for regenerative medicine.
In sickness and in health
Animal cells typically produce energy by breaking down oxygen. If oxygen is low, such as when exercising, cells can still produce energy by breaking down sugar in a process called glycolysis.
In a phenomenon known as the Warburg effect, most cancer cells ramp up their use of glycolysis even when oxygen is available.
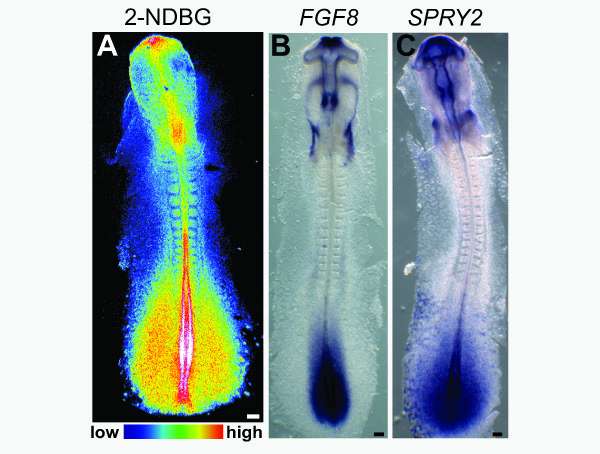
Scientists have also been aware of Warburg-like metabolic activity at certain stages of embryonic development, but up until now, its purpose in normal and cancerous cells remained murky.
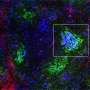
Pourquié's team, which included collaborators from the Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) in France, sought answers by examining Warburg-like glycolysis in chick and mouse embryos at a resolution not seen before.
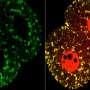
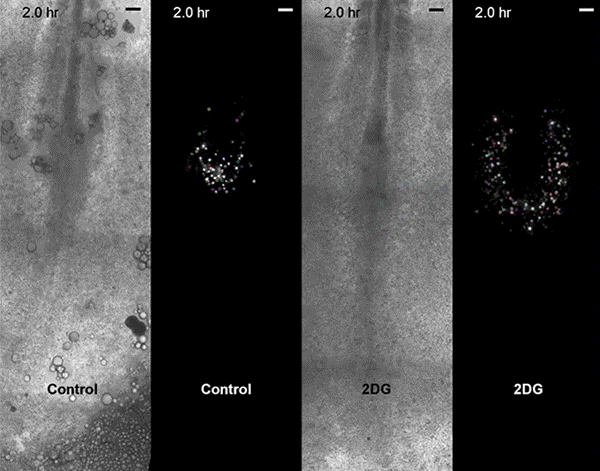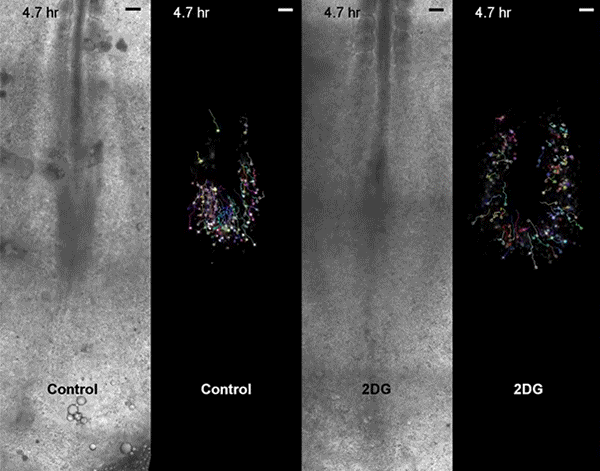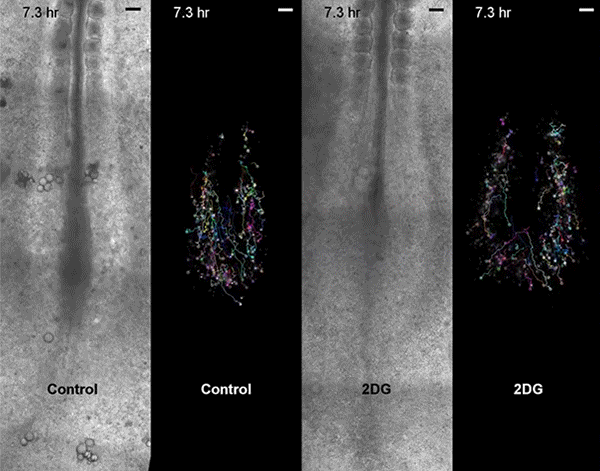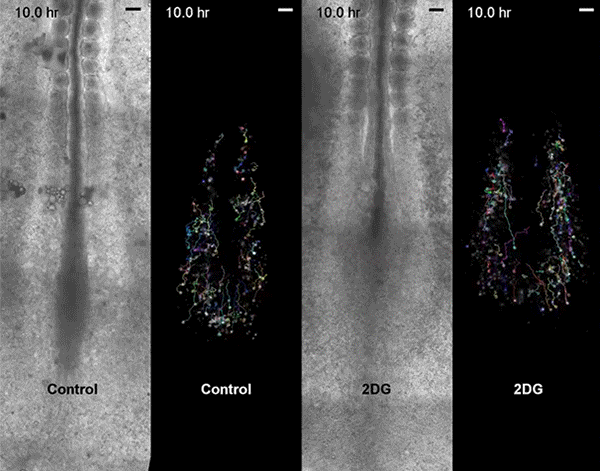
The first surprise arrived when the researchers found that glycolysis peaked along the spinelike axis in the tail bud, where the embryo was busy growing longer, and faded toward the middle, or trunk, of the embryo.
Then came the second unexpected discovery. Although biologists had proposed that the Warburg effect helps cancer cells proliferate, glycolysis in the embryos didn't map onto cell proliferation. Instead, high glycolysis coincided with high cell signaling.
Specifically, glycolysis spiked where cells were busy speaking in two "languages," FGF (fibroblast growth factor) and Wnt signaling. These signals control cell growth and the differentiation of so-called paraxial mesoderm cells into muscle and skeletal tissues. They also tend to get thrown out of whack in many cancers.
Pourquié and team noticed another metabolic parallel between the embryos and cancer cells: Regions of high glycolysis had low, or acidic, extracellular pH, "which is exactly what you would see in many tumors," he said.
"Many aspects of the embryos' metabolism resemble cancer cells," said Pourquié. "I was quite surprised by how similar they are."
The resemblance raises hopes that learning what Warburg-like metabolism does in developing embryos will illuminate its role in cancer and perhaps, one day, point to new avenues for cancer treatment.
"People have said for years that cancer cells have an embryonic phenotype," said Pourquié. "Here we provide a detailed metabolic analysis to show that we can push the analogy with cancer cells very far."
The results lend support to the theory that the Warburg effect arises because cancer cells "reenact an embryonic program that is present when cells are proliferating and growing," he said, rather than the theory that their metabolism "is completely messed up."
But only time and further experiments will tell.
"Metabolism is very complicated, so to incorporate it into our studies is going to be a lot of work," said Pourquié. "But that's exciting."
More information: Masayuki Oginuma et al. A Gradient of Glycolytic Activity Coordinates FGF and Wnt Signaling during Elongation of the Body Axis in Amniote Embryos, Developmental Cell (2017). DOI: 10.1016/j.devcel.2017.02.001