Gut bacteria give newborns infection protection, not just digestion, mouse study shows
Hundreds of thousands of babies worldwide die every year from infections that ravage their digestive systems - including those caused by Salmonella and E. coli bacteria. Millions more get sick.
Could the difference in survival come not from their immature immune systems, but rather from the mix of bacteria that grow in their tiny guts?
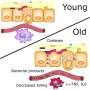
New research in mice offers evidence that some of those bacteria - called Clostridia—provide key protection against infection, in addition to helping digest food. But it also shows that the youngest newborn mice don't have Clostridia yet, making them the most vulnerable to invading bacteria similar to the pathogens that sicken so many human babies.
The findings, made at the University of Michigan Medical School and published in Science, could point the way to new approaches to protect human babies.
"Any parent knows that newborns are very susceptible to infections in the first year of life, including enteric, or gut, infections," says Gabriel Nunez, M.D., the study's senior author and a U-M pathology professor. "This work suggests that the lack of protective bacteria in the gut microbiota is a mechanism for that susceptibility, perhaps more than the age of the immune system."
Nunez and his colleagues, including co-first authors and research fellows Yun-Gi Kim, Ph.D. and Kei Sakamoto M. D., Ph. D., started with a blank slate: mice bred in a germ-free environment at U-M.
With no natural gut bacteria of their own, the mice offered a unique chance to see the effects of transplanted microbes from normal mice of different ages, and to test vulnerability to infection. The researchers also used advanced DNA analysis techniques that allowed them to detect the types and amounts of bacteria in mouse guts.
The bottom line of their experiments: Somewhere in the period around weaning mice from mother's milk to solid food, Clostridia bacteria begin to grow in the gut, and work to prevent the growth of two forms of illness-causing bacteria.
A series of experiments
Nunez, Kim, Sakamoto and their colleagues carried out a careful series of experiments using both newborn and adult germ-free mice, and samples of gut microbes taken from the feces of 4-day-old, 12-day-old and 16-day-old normal mice.
They found that the samples from the older normal mice had the most diversity of their gut microbes, including Clostridia and Bacteroides bacteria not seen in the younger mice that were still getting their nutrition entirely from mother's milk.
First, they gave the germ-free mice a transplant of bacteria from 4-day-old or 16-day-old normal mice, and then exposed them to a strain of Salmonella that can infect the gut but not spread body-wide. Half the mice that got the 4-day microbes died, but none of those with 16-day microbes did.
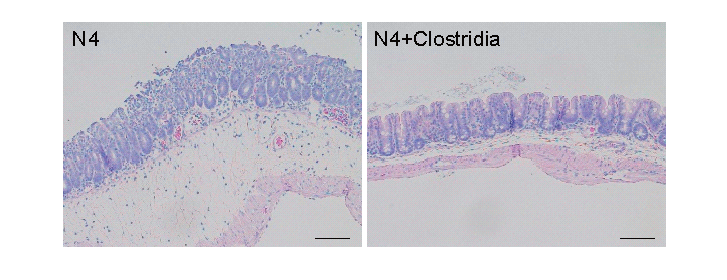
They tried it again with Citrobacter rodentium - a strain of bacteria similar to the E. coli strains that make humans sick. Germ-free mice with transplanted 4-day microbes got sick and many died. But when the researchers added bacteria from 16-day-old normal mice, the amount of C. rodentium in the guts of surviving mice went down.
Next, the researchers looked at what happened to germ-free mice that had been given a newborn mouse's microbes, but with extra doses of either Clostridia or Bacteroides bacteria added in. They exposed groups of these mice to C. rodentium - and found that only the mice given Clostridia were able to resist the infections. Ninety percent of the mice that got extra Clostridia, then Salmonella, were still alive after a week, compared with 50 percent of those that hadn't received it.
Since E. coli and Salmonella also affect adults, the researchers tested what happened when normal adult mice were given vancomycin, an antibiotic that selectively kills bacteria like Clostridia and Bacteroides. Both C. rodentium and Salmonella flourished in these environments.
To see what role the body's own immune system played in fighting infection, compared with gut microbes, the team also studied two strains of mice that have impaired immune systems. Raised in a germ-free environment, and then given a transplant of gut microbes from a four-day-old normal mouse, these mouse were still able to resist Salmonella infection without any help from their immune system - but only when they had received a dose of added Clostridium first.
Finally, the researchers looked at the impact of adding succinate - a salt that oxygen-loving bacteria in the gut produce as a byproduct - into the drinking water of germ-free mice with 4-day microbes that had received extra Clostridia.
These mice fought off Salmonella infection even better - suggesting that the anaerobic Clostridia feed off the waste products of the aerobic bacteria that flourish in the guts of newborns.
Nunez and his colleagues are already working on further research on the role of Clostridia in defending against gut infections. They want to determine which strains of Clostridia - and there are many - have the largest effect.
They're also looking at the role of mother's milk in establishing a newborn's gut microbiome and conveying protection from infection, as well as the transition to solid foods that can carry microbes into a newborn's gut from the outside world. And, they want to test whether other components of the microbiome protect against other pathogens.
"Normally, we acquire Clostridia strains in our guts when we begin to eat solids, but this work suggests a window of vulnerability to enteric pathogens in the early stages of life," says Nunez, who holds the Paul deKruif professorship in pathology and is a member of the executive committee for the U-M Medical School's Host Microbiome Initiative. He notes that the research would have been impossible without the Medical School's Germ-Free Mouse Facility.
He says that if the protective role of added Clostridia for newborns bears out in further animal studies, it might be possible to propose a clinical trial in humans to test a combination of strains.
More information: "Neonatal acquisition of Clostridia species protects against colonization by bacterial pathogens," Science (2017). science.sciencemag.org/cgi/doi … 1126/science.aag2029