First participant treated in trial of stem-cell therapy for heart failure
A research team at University of Wisconsin School of Medicine and Public Health has treated its first patient in an innovative clinical trial using stem cells for the treatment of heart failure that develops after a heart attack.
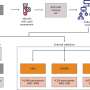
The trial is taking place at University Hospital, one of three sites nationwide currently enrolling participants. The investigational CardiAMP therapy is designed to deliver a high dose of a patient's own bone-marrow cells directly to the point of cardiac injury to potentially stimulate the body's natural healing response.
The patient experience with the trial begins with a cell-potency screening test. Patients who qualify for therapy are scheduled for a bone-marrow aspiration. The bone marrow is then processed on-site and subsequently delivered directly to the damaged regions in a patient's heart in a minimally invasive procedure.
"Patients living with heart failure experience a variety of negative symptoms that can greatly impact their day-to-day life," said UW Health cardiologist Dr. Amish Raval, associate professor of medicine and one of the principal investigators for the trial. "By being at the forefront of research for this debilitating condition, we look forward to studying the potential of this cell therapy to impact a patient's exercise capacity and quality of life."
The primary outcome to be measured is the change in distance during a six-minute walk 12 months after the initial baseline measurement.
Heart failure commonly occurs after a heart attack, when the heart muscle is weakened and cannot pump enough blood to meet the body's needs for blood and oxygen. About 790,000 people in the U.S. have heart attacks each year. The number of adults living with heart failure increased from about 5.7 million (2009-2012) to about 6.5 million (2011-2014), and the number of adults diagnosed with heart failure is expected to dramatically rise by 46 percent by the year 2030, according to the American Heart Association (AHA).
The CardiAMP Heart Failure Trial is a phase III study of up to 260 patients at up to 40 centers nationwide. Phase III trials are conducted to measure effectiveness of the intervention, monitor side effects and gather information for future use of the procedure. Study subjects must be diagnosed with New York Heart Association (NYHA) Class II or III heart failure as a result of a previous heart attack.
Information about eligibility or enrollment in the trial is available at www.clinicaltrials.gov, or through a cardiologist.