Immune responses from early study of novel sarcoma vaccine
The critical component of an experimental vaccine led to an escalating immune response in patients with sarcoma, an indicator of its potential anti-cancer effects.
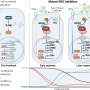
The findings give hope for a vaccine for sarcoma, called CMB305, an immunotherapy strategy that involves using an engineered virus to teach patients' immune systems to recognize and kill tumor cells.
The findings will be presented by Dr. Seth Pollack, a physician-scientist at Fred Hutchinson Cancer Research Center, June 5 in a poster at the American Society of Clinical Oncology annual meeting in Chicago.
The poster presentation reinforces the results of an early-stage clinical trial led by Dr. Neeta Somaiah of MD Anderson Cancer Center and to be presented June 2 at the ASCO meeting. Pollack is a senior investigator on the trial.
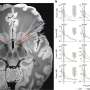
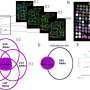
In the poster presentation, Pollack—a physician-scientist who specializes in sarcoma—and his collaborators examined participants' immune responses before and after receiving the vaccine. They studied 62 patients enrolled in this trial or in an earlier trial that used only the virus component of the vaccine without the additional immune-boosters included in CMB305.
The data suggested that the vaccine was working as designed: In patients on both trials, Pollack and his collaborators found multiple signs of escalating immune responses to NY-ESO-1, a protein marker found almost exclusively in cancer cells, but not healthy cells. An immune response directed at NY-ESO-1 will kill cancer cells but leave healthy cells alone.
Patients' immune systems also started recognizing and responding to other cancer markers besides NY-ESO-1 after vaccination, too.
But across the board, Pollack's team observed the biggest boost in signs of anti-cancer immunity among patients vaccinated with the newer version of the vaccine, CMB305.
"My feeling is that [the additional component in the CMB305 vaccine] does help, because we definitely saw a better immune monitoring response," Pollack said.
In the June 2 ASCO presentation of the Phase 1 clinical trial results, Somaiah will report that in 16 of 25 participants with advanced, soft-tissue sarcoma, tumors stopped growing after the patients received injections of CMB305. Of these, about three-quarters had no disease progression by three months, 36 percent had no disease at six months and 83 percent of them were still alive after one year.
The results "compare favorably" to published data on several FDA-approved therapies for these cancers, Pollack said.
Patients received four injections of the engineered virus over three months, plus a series of injections of an immune-boosting formulation for a year. The two subtypes of sarcoma studied in this trial were myxoid/round cell liposarcoma and synovial sarcoma.
"The results, so far, are exciting and show that the vaccine works; generates an immune response and stabilizes tumors, and will definitely lead to additional studies," said Somaiah, who led the study. "Hopefully, if we design the studies right, we will have this as a treatment option in the near future."
One trial participant had a serious side effect (severe pain); the rest reported relatively minor side effects like soreness at the injection site that lasted one day.
"The lack of side effects makes the findings from the CMB305 trial especially noteworthy," Pollack said. "For most patients with these sarcomas, the most difficult symptoms they face throughout their illness are side effects from chemotherapy, which is a typical treatment for sarcomas."
The engineered virus in CMB305, which incorporates elements of multiple natural viruses, derives from discoveries made in the lab of Nobel Prize-winning scientist Dr. David Baltimore of CalTech. Baltimore and the Hutch's Dr. Larry Corey are among the scientific co-founders of Seattle's Immune Design, which owns CMB305 and sponsored this trial.