How anesthetics work, and why xenon's perfect

Common wisdom maintains that, because of the myriad effects on the brain, how anesthetic drugs work at the molecular level is a mystery.
On the contrary. As a longtime pharmacology researcher, I believe there's a sufficient body of evidence to certify it's not so mysterious after all.
First, some information —and a bit of a history lesson —on anesthetics for all the armchair scientists and doctors among us.
General anesthetics are so called because the administered drug is transported via the blood throughout the body, including the brain, the intended target.
The first general anesthetic used clinically was nitrous oxide, a gas synthesized in a research lab in 1772. It's still known as laughing gas, and in later years, because it could not silence the brain sufficiently, it was useful only for minor surgery.
By the 1800s, William T.G. Morton (1819-1868), a young Boston dentist, was on the hunt for a better anesthetic than nitrous oxide, commonly used then by dentists.
Ether was a liquid compound produced by distilling ethanol and sulfuric acid. It was just a curiosity at the time. But Morton left a bottle of ether open in his living room and passed out. In 1846, he gave the first public demonstration of ether's effects on a patient undergoing major surgery.
How it works
How do general anesthetics like ether work to subdue brain function?
Most are inhaled and administered from pressure tanks. Ether, as a liquid, emits vapours that are inhaled. Another extremely potent liquid anesthetic is propofol, administered intravenously. It was identified as a major contributor to pop icon Michael Jackson's death.
Some barbiturates given via IV are useful general anesthetics. Alcohol is another, but it's too toxic for clinical use.
The process of anesthesia is commonly divided into four stages.
The four phases of unconsciousness
- Stage 1 is known as induction, the period between the administration of anesthetic and loss of consciousness.
- Stage 2 is the excitement stage, the period following loss of consciousness and marked by excited and delirious activity.
- Stage 3 is surgical anesthesia. Skeletal muscles relax, vomiting stops if present, respiratory depression and eye movements stop. The patient is ready for surgery.
- Stage 4 is overdose, involving severe depression of vital organs that can be lethal.
Works in worms like it works in humans
The various compounds that produce anesthesia in human beings do so in all animals, including invertebrates. The response of the earthworm, C. elegans, to the steady administration of anesthetic elicits a progressive depression of function similar to how it works in humans.
There is an initial phase of increased locomotion, followed by uncoordination, and finally immobility. Motion returns quickly when the administration of the anesthetic stops. This shows that optimal nerve cell architecture developed early in the evolution of life on Earth.
But now let's do a deep dive into what happens at the molecular level. How does the anesthetic molecule obstruct vital molecules or molecule assemblies essential for cell function in order to bring about unconsciousness?
A prevalent lipid (fat) theory of anesthetic action had been based on the fact that all anesthetics are "hydrophobic" chemical compounds, meaning they mix with oil but not water. Presumably, they impair brain cell (neuron) function and bring about unconsciousness by dissolving into the fatty cell membranes, thereby disrupting normal cell activity.
I doubted this theory.
Proteins are critical to understanding anesthesia
And so 35 years ago, I made the observation that the molecular weights of the different anesthetics were no more than about 350 Daltons, comparable in size to the smaller messenger molecules that activate the utilitarian proteins in cells.
Functional, vital proteins are the cell's workhorses. They include receptors that serve to communicate to the cell signals from hormones and other regulators that induce changes in cell activity in a variety of ways, and ion channels that constantly monitor and control the cells' levels of sodium, potassium and calcium, a process particularly vital for brain cell function.
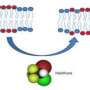
The proteins are spherical and contain at their cores a cavity lined with hydrophobic parts (those that mix with oil, not water) of the surrounding constituent amino acids, and they accommodate small so-called regulator molecules.
The cavities are about the same size for all these proteins, but differ from one another only by the types of constituent amino acids both lining and around the cavity.
Cavity penetration sets off chain of events
An estimated volume for the cavity reported for one particular type of protein ranged from 853 to 1,566 cubic Angstroms. By way of comparison, the volume of an occupant of the cavity, the epilepsy drug diphenylhydantoin (brand name Dilantin, used to control seizures) was reported as 693 cubic Angstroms —small enough to occupy the cavity, as all anesthetics are.
The penetration into the cavity by the anesthetic molecule causes the protein to activate an intracellular process, or the opening of an ion channel that, as mentioned, controls the cell's levels of sodium, potassium and calcium. And unconsciousness begins.
Is There a General Anesthesia Receptor? That's the title of a paper I published in 1982. The answer is: Yes, there is a general anesthesia receptor. It's the crucial central cavity in all vital cell proteins.
The many cellular vital proteins and their small regulator molecules constitute a biological lock-and-key, each with its own special key. The anesthetic molecule occupies all locks, thereby obstructing all keys.
Today, it's generally accepted that proteins are the targets of general anesthetics and that the lipid theory is ancient history.
So what's the perfect anesthetic?
The diverse molecular structures of anesthetics are reflected in their different repertoires of interactions with numerous protein cavities and other cellular entities. That means each anesthetic is unique in how it precisely sedates patients, and has unique side effects.
The ideal anesthetic would have these major characteristics: chemical stability, low flammability, lack of irritation to airway passages, low blood:gas solubility to allow for patients to be sedated and brought out of sedation quickly, minimal cardiovascular and respiratory side effects, minimal effect on brain blood flow and low interactions with other administered drugs.
In the operating room, the agent that ticks all those boxes is the gaseous xenon atom.
Xenon is one of the mono-atomic rare, "noble" gases present in trace amounts in the atmosphere. The others are helium, neon, argon, krypton and radon. They are inert, meaning they have extremely low chemical reactivity.
Xenon's sole interaction with biological tissue is the occupation of protein cavities. The xenon atom is like a smooth, round billiard ball and has no appendages to engage other entities —a phenomenon that accounts for many of the side effects of other anesthetics. The xenon atom literally just rolls into a protein cavity and does not interact with anything else in the cell.
The gas is unique. Side effects are almost non-existent.
Inhaled, blood-borne xenon permeates body tissues harmlessly until it engages a protein pocket, where it becomes entrapped. The amino acids lining the cavity then form a tight bond with xenon.
Xenon: a noble gas, an ideal and noble anesthetic
As a result, xenon shuts out the physiological activator molecule, leading to the shutdown of the vital protein and, thus, impairment of cell function. All of that amounts to a safely and efficiently unconscious patient.
So why isn't xenon the anesthetic of choice for surgery in general?
A chief factor is its steep pricetag. There have been attempts to overcome that hurdle by, for example, installing devices to recover the exhaled xenon in the operating room atmosphere after it's been administered to a patient; xenon recycling, so to speak.
That's a challenge. The next formidable challenge in our understanding of anesthetics is figuring out which vital proteins in which brain neurons —among the billions of neurons —are silenced in turn with progressively deeper anesthesia.
But, optimistically, that can be the subject of a future science lesson.
This article was originally published on The Conversation. Read the original article.![]()