The importance of randomised control trials in medical research
When a new treatment becomes available for a particular health condition, such as a new medication to treat a disease, it's tested to see whether it's effective for its intended purpose. It's also tested for potential side effects. This is done through a series of human trials, known as clinical trials.
Every day hundreds of people are invited to participate in clinical trials to test new treatments. Clinical trials are conducted across four phases, from early studies to test the treatment's safety in a small group of people (phase one), to much larger studies testing whether the treatment works in patients (phases three and four). People are invited to participate in all of these phases.
Once a new treatment is found to be safe (in terms of having acceptable side effects) in the first two phases, it's assessed for whether it is beneficial to patients compared with the standard treatment for that condition (phase three). This information can then be used to change clinical practice.
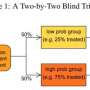
The best way to compare a new treatment to the standard treatment is in a randomised controlled trial. In such a study, participants are randomly allocated to either the new or standard (control) treatments using the computer equivalent of tossing a coin. This process is known as randomisation and is the only commonly accepted method of ensuring an unbiased estimate of the treatment effect.
Why randomise?
For the comparison between the new and standard treatment to be fair, the patients receiving the different treatments need to have similar characteristics. Imagine if the group receiving the standard treatment was made up only of children, while only adults received the new treatment. Then you couldn't tell whether the way the treatment affected people in either group was due to the treatment itself or the physiological differences between the children and adults.
This confusion of effects is known as confounding, and randomisation is used to overcome it. In randomised trials, treatments are randomly allocated to the trial participants, so each individual has the same chance of receiving the new or standard treatment. Randomisation ensures the the people in each group have a similar distribution of characteristics to make sure that the groups are comparable - so if one group has better outcomes than the other, it can be attributed to the interventions being tested.
Randomisation is therefore critical to make direct comparisons between the treatments. It is also important that the treatment allocation is concealed from both the patient and doctor before the patient joins the study so that this cannot influence the decision for the patient to take part in the study.
Control group
The control group is the one the new treatment is being compared with. So, people in this group generally receive the standard treatment for treating the disease of interest. If there is no standard treatment, the control group may receive either no treatment at all or a placebo (an identical treatment but with no active ingredient).
The presence of a control group is important to understand what happens to similar patients who undergo the same procedures as in the other group(s), but in the absence of the new treatment.
Blinding
Ideally in a randomised controlled trial, the treatment is masked from the patient and the medical staff treating the participant. This is known as blinding.
If the participant knows they are receiving a new treatment, they may have certain expectations which could lead them to actually feel better - this phenomenon is known as the placebo effect. Similarly, if the treating doctor knows the participant is receiving the new treatment, they may interpret the participant's progress more positively than for a participant receiving the standard treatment.
Blinding both the participant and all the medical staff involved in a trial ensures that the participant's progress is measured independent of the treatment being received. This removes the potential for bias due to the participant or doctor's perception of the treatments being compared.
In some situations, it's not possible to blind the participant or the treating medical staff to the treatment allocation. For example, it would not be possible to blind participants in a trial comparing plaster casts and removable splint for wrist fractures. In this case it's important to blind the people assessing the outcome, where possible.
Why agree to be in a trial?
As when tossing a coin, in most trials participants have a 50% chance of receiving each of the two interventions. Only having a 50% chance of getting a new treatment may not sound appealing, but it's important to note trials are only conducted when it's unknown whether the new treatment is beneficial over the standard treatment. It's actually possible the standard treatment may be better.
This state of not knowing which treatment is best is known as clinical equipoise, and means that no participant in a trial is knowingly getting an inferior treatment. This also applies when the control group receives a placebo, as it may be that the treatment does not have any benefit for the patient but may have unwanted side effects.
The advantages of taking part in a randomised trial are that you may receive a treatment that is better than standard care, and that you are often more closely monitored than under routine care outside of the trial setting.
For some health conditions, participating in a clinical trial provides the only chance to receive a potentially beneficial treatment that may take years to become commercially available. An altruistic advantage is that the results of the trial will be used to guide clinical practice that will benefit patients in the future.
This article was originally published on The Conversation. Read the original article.![]()