FDA to crack down on risky stem cell offerings
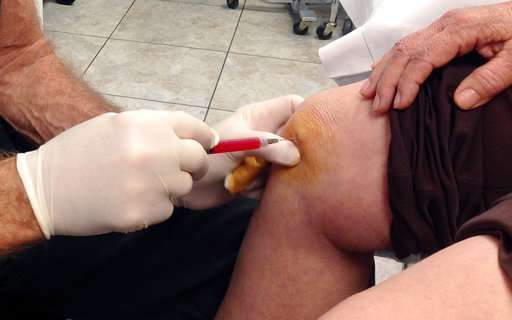
U.S. health authorities announced plans Thursday to crack down on doctors pushing stem cell procedures that pose the gravest risks to patients amid an effort to police a burgeoning medical field that previously has received little oversight.
The Food and Drug Administration laid out a strategy for regulating cell-based medicine, including hundreds of private clinics that have opened across the nation in the last decade. Many of the businesses promote stem cell injections for dozens of diseases including arthritis, multiple sclerosis and even Alzheimer's. They can cost $5,000 to $50,000, but there's little research that such procedures are safe or effective.
Researchers for years have called for a crackdown. FDA officials said they will focus their enforcement efforts on "bad actors" who inject stem cell mixtures into the bloodstream, nervous system or eyes. Regulators say those procedures pose the biggest risk to patients.
"We're going to be prioritizing places where we see products—not just being promoted inappropriately—but putting patients at potential risk," FDA Commissioner Scott Gottlieb told reporters on a conference call.
Gottlieb said the agency plans to use discretion in overseeing lower-risk procedures such as injections for achy joints, adding that this approach would allow the agency to get the "most bang for our regulatory buck." He also said the agency needs to be "nimble and creative" in its regulation to encourage legitimate researchers in the field.
Stem cells have long been recognized for their ability to reproduce and regenerate tissue. And while emerging research suggests that they will eventually be used to treat a range of debilitating diseases, they are currently only approved for a handful of medical procedures. For instance, adult stem cells from bone marrow transplants have long been used to treat leukemia and other blood diseases.
Most of the new clinics offer adults stem cells isolated from fat. Practitioners collect the fluid from patients via liposuction, treat it with chemicals and then inject it back into the body to treat various conditions.
Three Florida women were left nearly or completely blind by one such fat-based procedure, according to a report published earlier this year in the New England Journal of Medicine. The Florida Medical board previously revoked the license of another stem cell practitioner after two patients died under his care after receiving IV drips of stem cells to the bloodstream.
In August the FDA took action against clinics in Florida and California. The agency issued a warning letter to Sunrise, Florida-based US Stem Cell Clinic for marketing unapproved procedures for heart disease, Parkinson's disease and other conditions. And U.S. marshals, under FDA instructions, seized vials of an unproven vaccine from StemImmune Inc. of San Diego.
The FDA's authority to regulate stem cell procedures is a murky area that has been debated for years.
Typically the agency does not regulate individual doctors or their in-office procedures, focusing instead on products developed by drug and medical device manufacturers. But FDA has asserted its authority in certain cases when doctors begin processing stem cells and marketing them to treat serious diseases.
Guidelines released by the agency Thursday aim to clarify how much processing cells can undergo before triggering FDA regulation.
© 2017 The Associated Press. All rights reserved.

















