New gene therapy transplantation technique could improve treatment of neurodegenerative diseases
A therapeutic technique to transplant blood-forming (hematopoietic) stem cells directly into the brain could herald a revolution in our approach to treating central nervous system diseases and neurodegenerative disorders.
The technique, which could be used to transplant donor-matched hematopoietic stem cells (HSCs) or a patient's own genetically-engineered HSCs into the brain, was reported in Science Advances today by researchers from the Dana-Farber/Boston Children's Cancer and Blood Disorders Center and the San Raffaele Telethon Institute for Gene Therapy.
In their study, the team tested the technique in a mouse model to treat lysosomal storage disorders, a group of severe metabolic disorders that affect the central nervous system.
The team's findings are groundbreaking because, until now, it was thought that HSCs—from a healthy, matched donor or a patient's own genetically-corrected cells—needed to be transplanted indirectly, through an intravenous line to the bloodstream.
Therapeutic success has then depended on those cells engrafting in a patient's bone marrow, maturing and naturally circulating into the brain, at a very slow and inefficient rate.
A race against time
But in children with lysosomal storage disorders, caused by enzyme imbalances that result in a dangerous build-up of lipids, carbohydrates or other materials in the body's cells, time is of the essence to stop the disease's progression.
"The main issue with the conventional HSC transplant strategy has been the length of time needed for the therapy to take effect in the brain," says Alessandra Biffi, MD, director of the gene therapy program at Dana-Farber/Boston Children's and the senior author of the new study.
"It can take up to a year for the genetically-engineered cell lineage to proliferate, spread and produce therapeutic effects in the brain—oftentimes, patients don't have the luxury of time to wait," Biffi adds.
Biffi and her team wanted to find a faster—and more direct—way to transplant therapeutic HSCs into the brain.
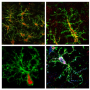
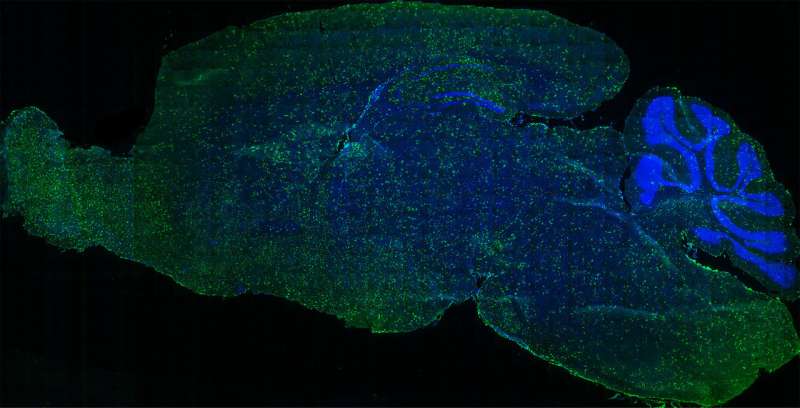
In a mouse model of lysosomal storage disorders, Biffi's team transplanted HSCs—which they had genetically engineered to correct the enzyme imbalance—directly into the brain. They found the direct approach jumpstarted the therapeutic benefits much faster than intravenous infusion alone. They call their method, which infuses the cells into fluid-filled cavities in the brain called ventricles, "intracerebroventricular" delivery.
Creating a chimera
Once the genetically-engineered HSCs are transplanted into the brain's ventricles, the crucial enzyme they contain helps to metabolize the materials that were previously building up and causing tissue damage.
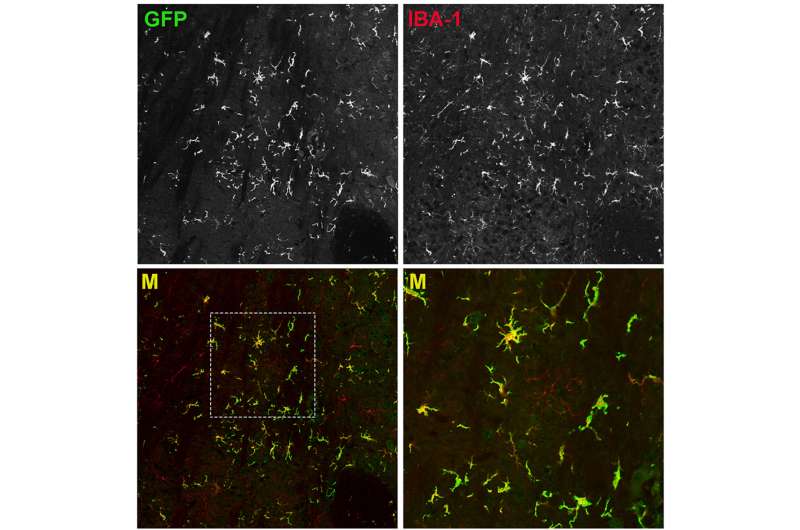
A new lineage of cells descended from the transplanted HSCs—a type of cell called a myeloid—begin to scavenge and consume the excess material that is responsible for neurodegeneration.
"There's a positive impact from the presence of the new, metabolically-functional myeloid cells because they release signaling cytokines that counteract neuroinflammation, which if unchecked can trigger neuronal damage," Biffi says.
Importantly, the transplanted HSCs engraft in the mouse brains without migrating to other areas of the central nervous system. This essentially could create a chimera—a separate genetic profile within an organism—within the brain.
The ability to engineer a chimeric population of brain cells could open powerful new avenues to preventing or reversing neurodegenerative diseases like Parkinson's, Alzheimer's, ALS and more.
From the lab to the human brain
Although transplanting HSCs directly into the human brain sounds invasive at first, Biffi explains that the procedure would not be overly complex in actuality.
"I envision this could be a one-time treatment accomplished via a catheter temporarily placed into the brain's ventricles, under standard anesthesia," Biffi says. "This would be in line with currently-used clinical procedures that enable access to the brain for treatment."
Based on the promising results of their mouse studies, Biffi and her colleagues are moving forward with plans to develop the procedure for the clinic.
She says there is great potential for intracerebroventricular delivery of genetically-modified HSCs, alone or in combination with intravenous gene therapies. This approach would be a new tool for clinicians to treat a range of conditions that affect the brain or the entire nervous system.
More information: A. Capotondo el al., "Intracerebroventricular delivery of hematopoietic progenitors results in rapid and robust engraftment of microglia-like cells," Science Advances (2017). DOI: 10.1126/sciadv.1701211 , advances.sciencemag.org/content/3/12/e1701211