US officials seek packaging fix for diarrhea-drug abuse

U.S. health regulators on Tuesday asked makers of popular anti-diarrhea drugs to sell their medications in smaller amounts to make them harder to abuse.
The request comes amid a spike in overdoses from large doses of the over-the-counter drugs, which contain a small amount of an opioid.
The Food and Drug Administration wants manufacturers to package their medications in smaller quantities, such as eight tablets per package. Currently, some generic versions are sold in boxes of up to 200 tablets. The FDA said it also plans to ask online retailers to make it harder to order bulk amounts of the drugs.
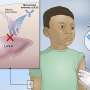
The key ingredient in anti-diarrhea medications like Imodium is part of the opioid family, an addictive drug class that includes morphine and oxycodone. At low doses, the medicine, known generically as loperamide, helps control diarrhea. But recent statistics show a rise in abuse of the drug, including massive doses that can cause heart problems and death.
In some reported cases, people attempted to wean themselves off opioids by substituting the anti-diarrhea drugs.
Fifteen deaths were tied to the drug between 2010 and 2016, researchers reported in a study last year.
Previously, some experts have called for sales restrictions on the medicine similar to pseudoephedrine, the decongestant in Sudafed that can be processed into methamphetamine. Pharmacies now keep Sudafed and related medicines behind the counter and limit purchases. But such restrictions generally require changes to federal law from Congress.
Imodium was first approved as a prescription medication in 1976 and became available without a prescription in 1988. It is one of the few over-the-counter medications for diarrhea.
The FDA sent letters to several manufacturers, including Imodium-maker Johnson & Johnson and various pharmacy chains that sell their own lower-cost versions. J&J said in a statement it would "continue to partner with the FDA and others to educate consumers" on safely using the drugs.
The FDA and other federal agencies have been struggling to curb a nationwide epidemic of opioid abuse. Although initially driven by prescription painkillers, most of the deaths now involve illegal drugs like heroin and fentanyl.
© 2018 The Associated Press. All rights reserved.