The genome guardian turns to the dark side: Opportunity for drug discovery against cancer?
Abnormal changes in the p53 protein are associated with many cancers. In fact, the gene that codes the p53 protein is the one most frequently mutated in human cancers. The protein, known as the guardian of the genome, has the main role of suppressing tumor formation and in so doing it blocks cancer development. But once mutated, the p53 protein not only stops working as expected, it also acquires new functions and characteristics that are catastrophic for the cell.
One of the characteristics observed in mutated p53 is the tendency to form amyloid aggregates, which are structures that no longer display the protein original 3-D conformation, stick together, and are resistant to degradation. These amyloid aggregates build up in the tissue, are very harmful, and present a pathogen-like behavior where mutants highjack normal counterparts and convert them into the amyloid form. These aggregates have been found in many cancer patients and in other protein-misfolding diseases such as Alzheimer's and Parkinson's.
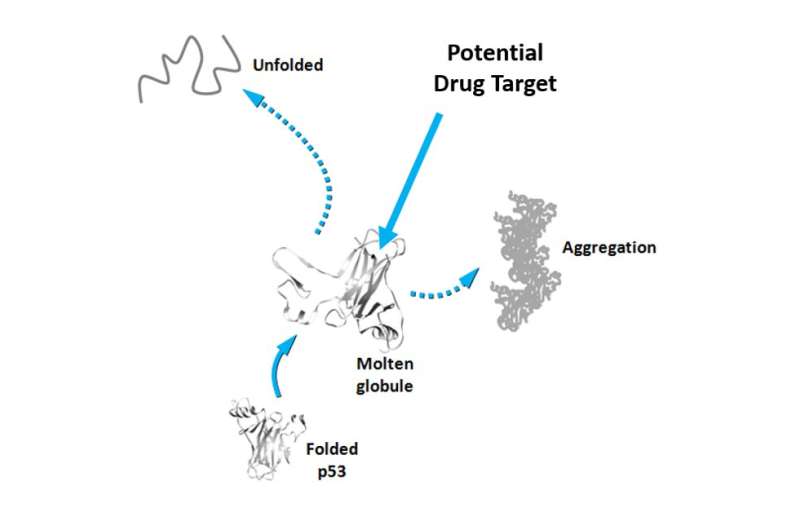
Somewhere along its transition from its native protein state to the deleterious amyloid form, p53 assumes a transient state as a molten globule (MG). MG-state p53, or pre-aggregate MG conformers, have not yet formed aggregates but are nevertheless very prone to doing so, which makes them a promising drug target. Thus, understanding the behavior of MG-state p53s and what prompts them into this conformation in the first place is crucial.
Targeting MG-states has been a real challenge because these states have a very short lifetime before they aggregate into amyloid structures. However, using a combination of techniques that include fluorescence spectroscopy, chemical and physical approaches, a research group led by Jerson Lima Silva at the Federal University of Rio de Janeiro, Brazil, has developed a new strategy that traps p53 conformers in solution so they can be observed before turning into amyloid oligomers and fibers. The group used the technique to study p53 and two other related proteins, p63 and p73. Of the three, p53 is the one that is least stable and most prone to aggregation.
While in real life the native state of p53 may be disrupted by the action of viruses (such as HPV), ultraviolet radiation (such as sun light) or exposure to carcinogens (tobacco smoke, nitropyrene emitted in diesel combustion, among many others), the research group applied different concentrations of chemicals and/or high hydrostatic pressure to disrupt the equilibrium of native proteins in the lab, as detected by fluorescence and nuclear magnetic resonance. The strategy is believed to recapitulate the same unfolding and aggregation processes that take place inside the body of cancer patients and allows researchers to get a closer look at the conformational changes suffered by individual amino acid residues before the molecule undergoes unfolding. This stage is a main step towards the amyloid state.
"A clear understanding of all the changes that occur in the molecule before it assumes the amyloid state may allow us to manipulate this process and have the option of either rescuing the native state of the p53 protein or blocking its transformation into amyloid oligomers and fibrils," says Guilherme A. de Oliveira, one of the study's co-authors.
In fact, both strategies have been previously used against mutated p53 by different groups but no clear results have been produced. With this study, the group presents a new technique for trapping p53 conformers in solution and claims that the p53 pre-amyloidogenic form is a promising alternative target for drug discovery.
The paper entitled "Aggregation-primed molten globule conformers of the p53 core domain provide potential tools for studying p53C aggregation in cancer" is published online in The Journal of Biological Chemistry.