Protein catch-22: Unravelling the roles of ataxin-2 in health and disease
Thousands of people took the Ice Bucket Challenge, a sensation in the summer of 2014. Participants were filmed dumping buckets of ice-cold water on their heads and challenging their friends to do the same. Challenged participants had 24 hours to comply or refuse, and thus forfeit a charitable financial donation. The point of the ice bucket challenge was to raise awareness of amyotrophic lateral sclerosis (ALS), a neurodegenerative disease afflicting an increasing number of people around the world. The event raised over $100 million U.S.
ALS affects nerve cells in the brain and spinal cord, causing muscle weakness. The disease makes it difficult to perform basic functions like walking, eating and speaking, and afflicted individuals can expect to live for two to five years. ALS is the notorious cousin of spinocerebellar ataxia type 2 (SCA2), an advancing, neurodegenerative, genetic disease that slows down the activity in the part of the brain that controls voluntary activities such as coordination, balance and speech. Patients with SCA2 may live for decades post-diagnosis, but the most severe cases can cause trouble with swallowing and breathing.
The World Health Organization, the World Bank, and the Harvard School of Public Health describe such neurological disorders as among the greatest burdens to public health, and they expect these diseases to advance to globally unmanageable levels in future.
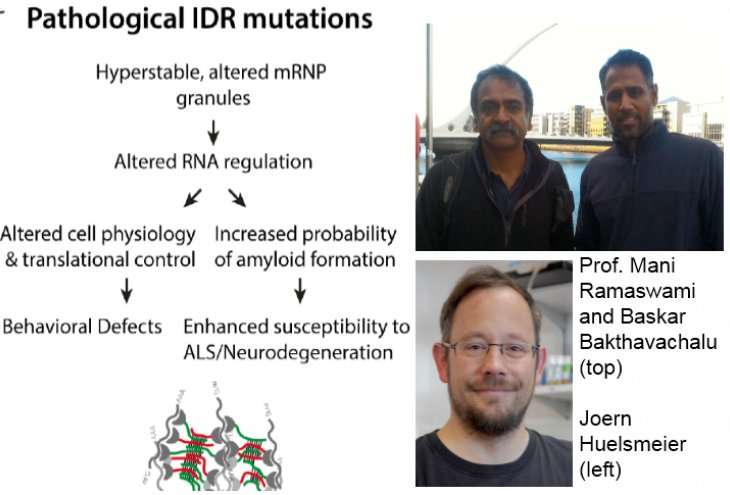
Novel scientific approaches are required to understand the risk factors resulting in the onset of such diseases as well as their progression and management to aid intervention and treatment. In this study led by Baskar Bakthavachalu (NCBS) and Joern Huelsmeier (Trinity College Dublin), recently published in Neuron, the team uncovered novel roles of a protein known as Ataxin-2 in the initiation and progression of ALS and SCA2.
Ataxin-2 is an essential protein of the nervous system. The researchers focused on a particular domain of Ataxin-2 known as the intrinsically disordered region (IDR), which was hitherto largely unknown. One of the predicted functions of IDR is to assist Ataxin-2 in the formation of granules inside the neuron. Knowing that Ataxin-2 and neurodegeneration were linked, the authors were interested to know if the IDR regions of Ataxin-2—and indirectly, the granules—had any role in promoting neurological dysfunctions. The study would have important bearings into the diverse scenarios disrupting normal brain functions.
These studies were performed using the fruit fly (Drosophila), as it has a single copy of the Ataxin-2 gene (in contrast to mammals, which have multiple copies). It was thus an ideal model for studying Ataxin-2 deletion.
Baskar and colleagues began their study by demonstrating that the IDR does indeed assist granule formation by controlling the stability and strength of Ataxin-2 interactions with other cellular components. This results in aggregation, a routine phenomenon. Flies with deleted Ataxin-2 IDR were completely normal, albeit deprived of their ability to form granules. Baskar says, "The main caveat in the field had been the lack of a system that could discriminate the function of the granule without interfering with the other important functions of Ataxin-2. We overcame this problem with a genetically engineered fly model in which these two described functions were separable."
Last year, two independent and largely similar studies demonstrated that reduced Ataxin-2 levels in mice predisposed them to ALS-like illness, and slowed down their onset and progression of neurodegeneration. Taking cues from these studies, Baskar and colleagues wondered if reduced neurodegeneration could be attributed to the lack of granule formation, or if it was a property of other functions of Ataxin-2. The finding that Ataxin-2 IDR-assisted granules facilitate its association with other pathogenic proteins (known to induce neurodegeneration), is a core highlight of this study. Thus, deletion of the IDR domain, thereby inhibiting granule formation, was sufficient to prevent neurotoxicity.
Importantly, the research also proved that Ataxin-2 contributed to long-term memory in flies via the IDR-granule connection. They also discovered that Ataxin-2 plays a vital role in the rewiring of the nervous system, a process known as "pathfinding"—which was unknown until now.
The study has conclusively shown that the IDR domain of Ataxin-2 allows the development of long-term memory, and paradoxically, also results in specific forms of neurodegeneration. How can an essential function such as maintenance of memory also be coupled to a devastating disease? Baskar says, "One possible explanation is that mechanisms that allow granule fluxes (formation and clearance) under normal scenarios are tilted towards granule accumulation in diseased conditions."
More information: Baskar Bakthavachalu et al, RNP-Granule Assembly via Ataxin-2 Disordered Domains Is Required for Long-Term Memory and Neurodegeneration, Neuron (2018). DOI: 10.1016/j.neuron.2018.04.032