Study examines safety and efficacy of TPA in mild stroke cases
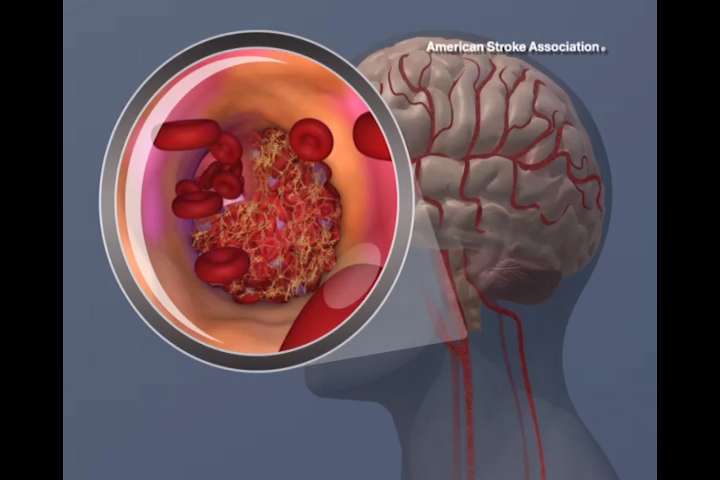
While the gold standard for stroke care is the use of IV tissue plasminogen activator (tPA, or product name Alteplase) to dissolve clots and mechanical thrombectomy to remove clots in severe to moderate strokes, researchers and clinicians have found it more difficult to align on a standard of treatment for mild strokes.
A national study looking at IV treatment of mild stroke, led by researchers at the University of Cincinnati (UC) College of Medicine, is published in the July 10, 2018 edition of the Journal of the American Medical Association.
Based on epidemiological data from the well-known Greater Cincinnati Stroke Study, about one-third of strokes are classified as mild stroke. Pooja Khatri, MD, professor of neurology at UC, and lead author on the study, wanted to determine if treatment with Alteplase showed benefit when extended to mild stroke.
Results of the study showed that use of tPA for mild stroke may not be as beneficial to the patient as was hypothesized.
"Mild stroke is the most commonly cited reason for deciding to not start tPA among patients who arrive to a hospital within the treatment window. But we also knew that after 90 day follow-ups, up to 30 percent of these patients would suffer from some kind of functional disability," says Khatri, who is also director of acute stroke care for UC Health.
"In this study, we wanted to evaluate the efficacy and safety of using tPA to treat patients of ischemic stroke only presenting with minor deficits, judged as not clearly disabling," says Khatri. "In Greater Cincinnati, I think our approach has been more conservative; we weren't always treating with alteplase (tPA), while some centers have been treating them very aggressively. Previous trials excluded these patients, so individual clinicians and stroke teams had to make their own clinical judgments about treatment." Khatri engaged with leaders at stroke centers across the country to better understand the treatment approaches for mild stroke.
The study used the National Institutes of Health Stroke Scale (NIHSS), which assesses stroke severity based on things like the patient's level of consciousness, facial droop and motor skills. A mild stroke is classified as 0 to 5, along with no clearly disabling deficits at time of presentation.
However, the trial ended early due to slower-than-expected enrollment. Outcomes for 313 patients were tracked and reported in this study, which does give researchers some evidence to consider when encountering mild stroke.
"Among the patients with minor, not clearly-disabling acute ischemic stroke, what we found is treatment with alteplase (compared with aspirin) did not increase the likelihood of a favorable functional outcome after 90 days," Khatri says.
She adds that the research team had hoped tPA would be beneficial in patients with a very mild neurological deficit.
"While the early termination does lead to uncertainty in the results, based on the data, it appears unlikely that the benefits of tPA extends to patients with mild stroke who do not present clearly disabling deficits," says Khatri, adding that this data provides additional information to physicians who treat stroke, and at some centers will be "practice-changing."
"[Here at UC] we were already on the side of not treating, but it is a bit more clear-cut to me now."
The study was funded by Genentech, makers of Alteplase. Khatri cites no conflict of interest.
Any stroke can have life-changing consequences, so it's important to identify the signs of a stroke which can be remembered with the mnemonic FAST: If you see Facial drooping, Arm weakness, or Speech is slurred, Time to call 911.