Alzheimer's treatment shows unique results
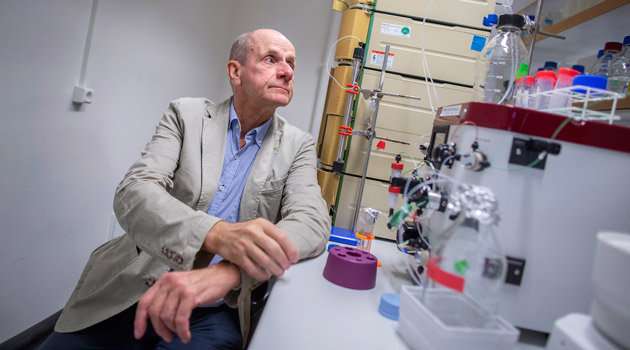
In late July there were reports in the media from a conference in Chicago about Alzheimer's disease. The focus of journalists' attention was a new study of how the progression of the disease is affected by the BAN2401 antibody, a Swedish product developed by Lars Lannfelt, Senior Professor of Geriatrics at the Department of Public Health and Caring Sciences.
"We have tested the antibody in various doses on 856 patients on three continents with better results than we even dared to hope for. After only six months we observed positive results, and after 18 months 81 per cent of the group receiving the highest dosage showed lower levels of amyloid beta than the level we measure in Alzheimer's disease," says Lannfelt.
A change in genetic material identified
The idea for the antibody originated back in the 1990s, when the team identified a change in the genetic material of a Swedish family that had been severely afflicted by Alzheimer's disease. The mutation indicated that the cause seemed to be a soluble, early form of amyloid beta molecules, known as protofibrils, which give rise to plaque in the brain.
"With this new knowledge, we chose to focus on these protofibrils, which we believed were the form of amyloid beta that causes the most damage. In 2005 we developed our antibody, and today I am extremely pleased with what we have achieved, for the sake of the patients and my many colleagues over the years and for Swedish research," says Lannfelt.
Modifications of the antibody
In 2003 Lannfelt and former research colleague Pär Gellerfors founded the BioArctic biotechnology company as a platform for pharmaceutical development. A significant part of the modifications of the antibody were carried out there, and when the results of the current study were made public, the shares soared in the stock market. Suddenly Lannfelt and Gellerfors saw their faces on Swedish business magazines accompanied by headlines such as "Swedish Alzheimer researchers became billionaires in two days".
"I do not see any problem with researchers benefiting from their innovations, but I find it annoying that the Swedish press has chosen to focus on the money rather than the medical innovation and its importance for patients. I have never been motivated by economic interests, and I will retain my shareholding to remain a guiding force in BioArctic's continued development and to help maintain the right course."
Today BioArctic is preparing for the Phase III study every pharmaceutical product must undergo before it reaches the market. At its side BioArctic has Eisai, Japan's pharmaceutical giant, which is assisting with funding and implementation. The goal is to go ahead as early as 2019, but despite BioArctic's successes, Lannfelt does not want to speculate on when an approved treatment can be available for health care.
"I have learned that it is far too easy to be optimistic about timing, but with the results we demonstrate in combination with minimal side effects, I believe we will be able to offer the first approved pharmaceutical for Alzheimer's disease. This would mean enormous socio-economic savings, and above all great hope for all the patients and relatives that we can help enjoy a better old age."