Biodegradable implant provides electrical stimulation that speeds nerve regeneration
Researchers at Northwestern University and Washington University School of Medicine have developed the first example of a bioelectronic medicine: an implantable, biodegradable wireless device that speeds nerve regeneration and improves the healing of a damaged nerve.
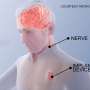
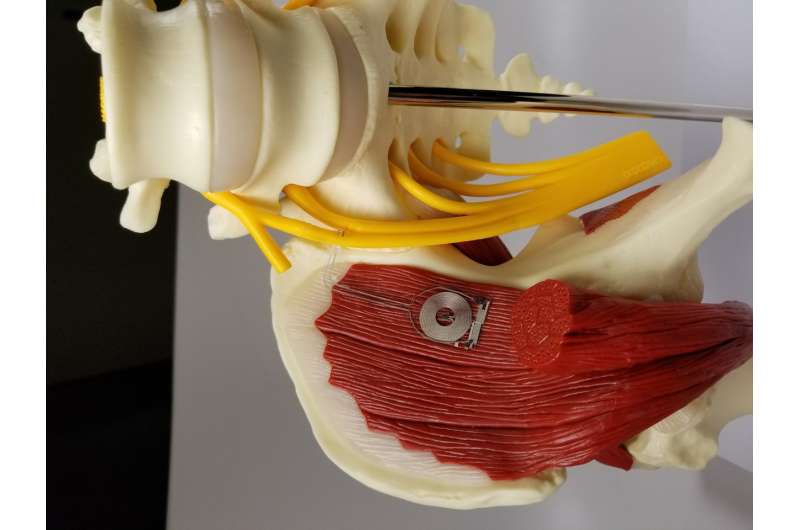
The collaborators—materials scientists and engineers at Northwestern and neurosurgeons at Washington University—developed a device that delivers regular pulses of electricity to damaged peripheral nerves in rats after a surgical repair process, accelerating the regrowth of nerves in their legs and enhancing the ultimate recovery of muscle strength and control. The size of a dime and the thickness of a sheet of paper, the wireless device operates for about two weeks before naturally absorbing into the body.
The scientists envision that such transient engineered technologies one day could complement or replace pharmaceutical treatments for a variety of medical conditions in humans. This type of technology, which the researchers refer to as a "bioelectronic medicine," provides therapy and treatment over a clinically relevant period of time and directly at the site where it's needed, thereby reducing side effects or risks associated with conventional, permanent implants.
"These engineered systems provide active, therapeutic function in a programmable, dosed format and then naturally disappear into the body, without a trace," said Northwestern's John A. Rogers, a pioneer in bio-integrated technologies and a co-senior author of the study. "This approach to therapy allows one to think about options that go beyond drugs and chemistry."
Rogers is the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery in the McCormick School of Engineering and Northwestern University Feinberg School of Medicine.
The research will be published Oct. 8 in the journal Nature Medicine.
While the device has not been tested in humans, the findings offer promise as a future therapeutic option for nerve injury patients. For cases requiring surgery, standard practice is to administer some electrical stimulation during the surgery to aid recovery. But until now, doctors have lacked a means to continuously provide that added boost at various time points throughout the recovery and healing process.
"We know that electrical stimulation during surgery helps, but once the surgery is over, the window for intervening is closed," said co-senior author Dr. Wilson "Zack" Ray, an associate professor of neurosurgery, of biomedical engineering and of orthopedic surgery at Washington University. "With this device, we've shown that electrical stimulation given on a scheduled basis can further enhance nerve recovery."
Over the past eight years, Rogers and his lab have developed a complete collection of electronic materials, device designs and manufacturing techniques for biodegradable devices with a broad range of options that offer the potential to address unmet medical needs. When Ray and his colleagues at Washington University identified the need for electrical stimulation-based therapies to accelerate wound healing, Rogers and colleagues at Northwestern went to their toolbox and set to work.
They designed and developed a thin, flexible device that wraps around an injured nerve and delivers electrical pulses at selected time points for days before the device harmlessly degrades in the body. The device is powered and controlled wirelessly by a transmitter outside the body that acts much like a cellphone-charging mat. Rogers and his team worked closely with the Washington University team throughout the development process and animal validation.
The Washington University researchers then studied the bioelectronic device in rats with injured sciatic nerves. This nerve sends signals up and down the legs and controls the hamstrings and muscles of the lower legs and feet. They used the device to provide one hour per day of electrical stimulation to the rats for one, three or six days or no electrical stimulation at all, and then monitored their recovery for the next 10 weeks.
They found that any electrical stimulation was better than none at all at helping the rats recover muscle mass and muscle strength. In addition, the more days of electrical stimulation the rats received, the more quickly and thoroughly they recovered nerve signaling and muscle strength. No adverse biological effects from the device and its reabsorption were found.
"Before we did this study, we weren't sure that longer stimulation would make a difference, and now that we know it does, we can start trying to find the ideal time frame to maximize recovery," Ray said. "Had we delivered electrical stimulation for 12 days instead of six, would there have been more therapeutic benefit? Maybe. We're looking into that now."
By varying the composition and thickness of the materials in the device, Rogers and colleagues can control the precise number of days it remains functional before being absorbed into the body. New versions can provide electrical pulses for weeks before degrading. The ability of the device to degrade in the body takes the place of a second surgery to remove a non-biodegradable device, thereby eliminating additional risk to the patient.
"We engineer the devices to disappear," Rogers said. "This notion of transient electronic devices has been a topic of deep interest in my group for nearly 10 years—a grand quest in materials science, in a sense. We are excited because we now have the pieces—the materials, the devices, the fabrication approaches, the system-level engineering concepts—to exploit these concepts in ways that could have relevance to grand challenges in human health."
The research study also showed the device can work as a temporary pacemaker and as an interface to the spinal cord and other stimulation sites across the body. These findings suggest broad utility, beyond just the peripheral nervous system.
More information: Jahyun Koo et al, Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy, Nature Medicine (2018). DOI: 10.1038/s41591-018-0196-2