Engineered probiotic strain of Escherichia coli bacteria can reverse dangerously high levels of blood ammonia
Synlogic, Inc., a clinical stage company applying synthetic biology to beneficial microbes to develop novel, living medicines, today announced the publication in Science Translational Medicine of clinical data from its Phase 1 clinical study in healthy volunteers and supporting preclinical data from its investigational Synthetic Biotic candidate, SYNB1020. The data support the continued development of SYNB1020 which is currently being evaluated in a Phase 1b/2a clinical trial in patients with cirrhosis and elevated blood ammonia.
"These data demonstrate that we can engineer bacteria to carry out a specific function, deliver them to humans and that they perform as designed," said Paul Miller, Ph.D., Synlogic's chief scientific officer. "Ongoing manufacturing and formulation development work at Synlogic gives us confidence we will be able to scale and formulate our Synthetic biotic medicines to meet multiple needs in the marketplace for living medicines. The compelling data in this publication encouraged us to advance SYNB1020 into additional clinical studies and we look forward to presenting data from our trial, designed to evaluate the potential of SYNB1020 to lower ammonia in patients with cirrhosis, in mid-2019."
Long-standing evidence supports the importance of the gastro-intestinal (GI) tract as the major source of ammonia in the systemic circulation. The current standard-of-care for conditions that result in hyperammonemia, including hepatic encephalopathy stemming from liver damage and urea cycle disorders (UCDs), which are genetic diseases, employ orally administered approaches such as antibiotics, laxatives and ammonia scavengers. However, each of these agents has limitations resulting in a need for additional therapies to manage hyperammonemia.
The publication titled, "An Engineered E. coli Nissle Improves Hyperammonemia and Survival in Mice and Shows Dose-dependent Exposure in Healthy Humans," describes the engineering and characterization of SYNB1020, preclinical studies of SYNB1020 in mouse models of hyperammonemia (OTC spfash and the TAA model), safety in healthy mice and non-human primates (NHPs) as well as clinical data from Synlogic's Phase 1 study of SYNB1020 in healthy volunteers.
Synlogic's Synthetic Biotic platform leverages the tools and principles of synthetic biology to engineer a strain of non-pathogenic bacteria (E. coli Nissle) to perform or deliver specific functions lost or damaged due to disease. Orally administered SYNB1020 has been designed to respond to the low oxygen environment of the large intestine to convert ammonia into arginine, an amino acid. In addition, Synthetic Biotic medicines are engineered to limit their replication after manufacturing so that they do not grow or colonize the GI tract.
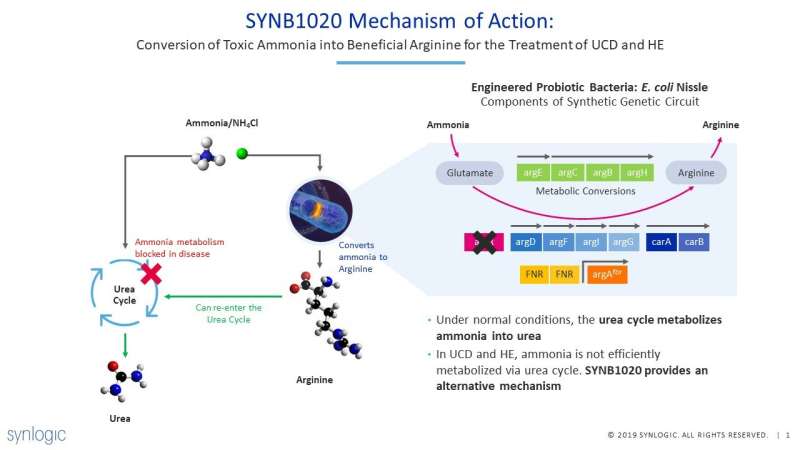
The preclinical data demonstrate that orally administered SYNB1020 is well tolerated in mice and NHPs, clears rapidly from the GI tract following completion of dosing and is not found in tissues outside the GI tract. When OTC spfash mice, a model of a urea cycle disorder (UCD), were fed a high protein diet to increase their blood ammonia levels, those that were orally dosed with SYNB1020 demonstrated lower blood ammonia and increased survival compared to mice that received heat-killed, inactive SYNB1020. Similar data were obtained in a mouse model of liver damage (TAA model).
The clinical data from Synlogic's Phase 1 clinical study demonstrate that, in healthy volunteers, orally administered SYNB1020 was safe, well tolerated, at daily doses up to 1.5 x1012 colony forming units, and cleared from the gastrointestinal tract within two weeks. In addition, dose-dependent elevation in plasma and urine of nitrate, a biomarker of SYNB1020 activity, was observed in healthy volunteers treated with SYNB1020 but not in those treated with placebo, demonstrating proof of mechanism.
Synlogic is currently evaluating SYNB1020 in a Phase 1b/2a clinical trial in patients with cirrhosis and elevated blood ammonia for the management of systemic ammonia levels and expects to report data in mid-2019. More information about Synlogic's Phase 1b/2a clinical trial can be found at https://clinicaltrials.gov under the study ID NCT03447730.
About Hyperammonemia
Hyperammonemia is a metabolic condition characterized by an excess of ammonia in the blood. In healthy individuals, ammonia is primarily produced in the intestine as a byproduct of protein metabolism. Ammonia is then converted to urea in the liver and is excreted in urine. However, if the liver's ability to convert ammonia to urea is compromised, either due to a genetic defect such as a urea cycle disorder (UCD) or acquired liver disease that leads to cirrhosis, ammonia accumulates in the blood. Elevated blood ammonia levels are toxic to the brain and can have severe consequences, including neurologic crises requiring hospitalization, irreversible cognitive damage and death.
More information: C.B. Kurtz el al., "An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans," Science Translational Medicine (2018). stm.sciencemag.org/lookup/doi/ … scitranslmed.aau7975