Immune cell therapy shows early promise for patients with pancreatic cancer

A nonengineered, multiantigen-specific T-cell therapy was safe, tolerable, and showed signs of clinical activity in patients who had pancreatic adenocarcinoma, according to preliminary results from a phase I clinical trial presented at the AACR special conference on Immune Cell Therapies for Cancer, held July 19–22.
"Pancreatic adenocarcinoma is extremely hard to treat," said Brandon G. Smaglo, MD, assistant professor of internal medicine and medical director of hematology/oncology at the Dan L Duncan Comprehensive Cancer Center of Baylor College of Medicine in Houston. "Most patients have intense chemotherapy, which often has severe adverse effects, and we urgently need alternative approaches to treatment.
"We are encouraged by the early results suggesting that the T-cell therapy we are testing is a feasible approach to pancreatic adenocarcinoma treatment that is tolerable and shows signs of clinical activity," continued Smaglo. "We look forward to treating more patients and continuing to follow those whose responses are ongoing."
Smaglo explained that certain proteins are specific to cancer cells and that these proteins are referred to as tumor-associated antigens. He and his colleagues set out to investigate whether it is possible to use a patient's blood to generate large numbers of T cells that recognize tumor-associated antigens, and that target and kill cancer cells once they are infused back into the patient.
To create each patient's customized treatment, the researchers start by harvesting immune cells from the patient's blood. T cells in the immune cell mixture that can recognize any of five tumor-associated antigens (PRAME, SSX2, MAGEA4, NY-ESO-1, and Survivin) are expanded in number in the laboratory under conditions that promote cancer-killing functionality and infused back into the patient.
As of July 5, 2019, 18 patients had been treated with up to six infusions of customized, nonengineered, multiantigen-specific T cells. Nine of the patients had unresectable or metastatic pancreatic adenocarcinoma that was responding to initial treatment with chemotherapy, and six had pancreatic adenocarcinoma that had progressed despite initial treatment with chemotherapy. The final three patients had pancreatic adenocarcinoma that could be removed by surgery. These patients received one T-cell therapy infusion before surgery and continued to receive T-cell therapy infusions after surgery; they are being followed for surveillance.
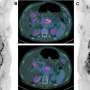
Seven of the nine patients who received the T-cell therapy alongside chemotherapy were evaluable for response. Five of those seven have had responses for longer than six months from the time of starting the T-cell therapy. The responses for these five patients are all ongoing. One of these responses is a radiographic complete response, two are partial responses, and four are stable disease.
One of the patients who had pancreatic adenocarcinoma that had progressed despite initial treatment with chemotherapy has stable disease that has been ongoing for more than six months since starting the T-cell therapy. Smaglo explained that while the others had progression of their disease, two had a clinical benefit with stabilization of their symptoms.
"We are excited that not only have we seen some clinical activity with our T-cell therapy, but we have seen no serious adverse effects, including no infusion-related systemic toxicity or neurotoxicity," said Smaglo. "The tolerability of the treatment is very important because other treatments for pancreatic adenocarcinoma can cause severe adverse effects."
According to Smaglo, the main limitation of the study is that only a small number of patients have been treated to date, so many more need to be treated to fully determine the effectiveness of the T-cell therapy. Smaglo also noted that generating the customized treatment is time and labor intensive for patients and health care providers, which may limit scalability of the T-cell therapy in the future.