Front-line osimertinib improves overall survival in EGFR-mutation positive NSCLC
First-line osimertinib significantly lengthens overall survival compared to older generation EGFR-TKIs in patients with Ex19del/L858R EGFR mutated advanced non-small cell lung cancer (NSCLC), according to late breaking results of the FLAURA trial presented at the ESMO Congress 2019 in Barcelona, Spain.
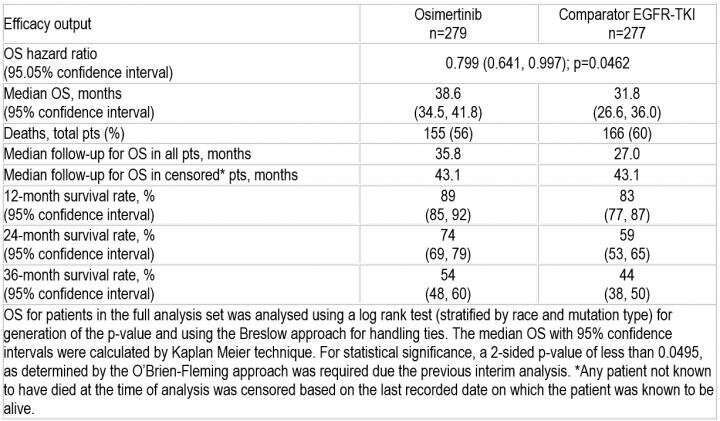
The primary endpoint of progression free survival (PFS) was previously reported. Survival data are now mature: the median overall survival with osimertinib was 38.6 months versus 31.8 months with first generation EGFR-TKIs, with a hazard ratio of 0.799 (p=0.0462). More than half (54%) of patients in the osimertinib group were alive at three years compared to 44% in the standard care group.
"The survival results are both statistically significant and clinically meaningful with first-line osimertinib for EGFR mutated patients," said study author Prof Suresh Ramalingam, Winship Cancer Institute of Emory University, Atlanta, US. "This is the first time a TKI has proven to extend survival relative to another TKI in lung cancer therapy."
Ramalingam noted that after disease progression, 31% of patients in the control group crossed over to the osimertinib arm, representing 47% of patients in the control group that received post-study therapy. "That is consistent with what we would expect in the real-world setting, since only about 50% of patients develop the T790M mutation and will be candidates for osimertinib," he said.
Ramalingam concluded: "FLAURA met both its primary and key secondary endpoints and showed a favourable safety profile for osimertinib. The results further reinforce the clinical utility and superiority of osimertinib in the front-line setting. Based on these data, osimertinib should be the preferred front-line therapy for EGFR-mutated lung cancer patients."
Commenting on the data, Dr. Pilar Garrido, Ramon y Cajal University Hospital, Madrid, Spain said the results with osimertinib in the first-line setting are good news for patients. She added that the magnitude of benefit in overall survival is also relevant for the debate about the best sequence of treatment since osimertinib is the only TKI approved for second-line treatment in patients who develop resistance due to T790M. She said: "If osimertinib is used as first-line therapy, there is no TKI available when the disease progresses. Patients should be told that osimertinib offers an overall survival advantage and is well tolerated, but when the treatment fails, the only option is chemotherapy. Maximising the duration of chemotherapy-free treatment is important for many patients, but if we want to know the most effective sequence of TKIs we need studies specifically designed for that."
More information: LBA5_PR 'Osimertinib vs comparator EGFR-TKI as first-line treatment for EGFRm advanced NSCLC (FLAURA): Final overall survival analysis' will be presented by Suresh S. Ramalingam during the Presidential Symposium I on Saturday, 28 September, 16:30 to 18:20 (CEST) in Barcelona Auditorium (Hall 2). Annals of Oncology, Volume 30, Supplement 5, October 2019.
Osimertinib is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) that targets both EGFR sensitising and EGFR T790M resistance mutations. It was approved for front-line use by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) after FLAURA met its primary endpoint of progression free survival. The primary results were presented at the ESMO Congress 2017 and subsequently published in the New England Journal of Medicine: Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113–125. DOI: 10.1056/NEJMoa1713137.