Treatment for leading cause of blindness generates billions in value to society

Researchers at the University of Southern California have developed an economic model to quantify the benefits of treatment for wet age-related macular degeneration (wAMD), the leading cause of blindness in western countries. Their work signals a step forward in the way ophthalmologists audit their practices to define the worth of modern treatments both to patients and society at large.
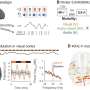
The study, led by Karen Mulligan, Ph.D. of the Sol Price School of Public Policy and the USC Schaeffer Center for Health Policy & Economics, Seth Seabury, Ph.D., director of the Keck-Schaeffer Initiative for Population Health Policy, and Mark Humayun, MD, Ph.D., director of the Dr. Allen and Charlotte Ginsburg Institute for Biomedical Therapeutics and co-director of the USC Roski Eye Institute, was published on November 14th in the Journal of the American Medical Association (JAMA). This research represents the first analysis to quantitatively compare the economic benefits of therapeutic injections for wAMD to their costs and assess the value they bring to society. The researchers' model showed that treating wAMD can generate $5.1 to $8.2 billion in patient benefit and $0.9 to $3 billion in societal benefit in three years based on the value of vision gains alone –– not including other potential benefits such as reductions in medical expenditure or caregiver burden. These numbers are poised to climb even higher if future innovations in health care can boost treatment initiation and adherence rates.
Quantifying therapeutic value
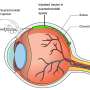
Wet age-related macular degeneration is a progressive form of blindness caused by the abnormal growth of blood vessels underneath the retina. A molecule called vascular endothelial growth factor (VEGF) drives this blood vessel formation, and doctors can treat the disease with a targeted therapy against that molecule to block blood vessel growth and restore patients' eyesight for up to five years. Anti-VEGF therapy is largely successful when injected into the eye regularly, yet many people with wAMD never initiate therapy and studies based on Medicare beneficiaries have shown that approximately 53-58% of wAMD patients receiving treatment discontinue within the first year.1,2 Cost, fear of discomfort related to the injection process and the time-consuming nature of follow-up care all contribute to this problem, prompting many doctors to modify treatment schedules to minimize the number of injections a patient must receive without drastically compromising effectiveness.
Finding the balance between providing enough treatment and minimizing the burden on patients can be tricky, and until now it was made even harder by the fact that no one had previously quantified the benefits that anti-VEGF therapy provides for patients and society in exchange for the costs associated with treatment. In an era when health care expenditure is growing at an unsustainable rate and policymakers are looking to cut costs where possible, this situation highlights the importance of quantifying which therapies are most worth their investments.
Other specialties have started a movement of quantifying treatment value and comparing benefits to costs, prompting Humayun to help pioneer this paradigm shift in ophthalmology by partnering with public policy experts like Mulligan and Seabury to audit the field's own practices.
An impressive return on investment
Humayun and his team started by combing through scientific literature and accruing model data of wAMD patients treated with anti-VEGF therapy. They translated the patients' changes in visual acuity over time to "quality-adjusted life years," which is an economic measurement that reflects both quantity and quality of years lived and could be used as a variable in the team's economic model.
"Our model assumes a person with perfect, 20/20 vision has a quality of life that is valued at $150,000 for a single year," Mulligan explains, referencing a commonly used value in health economics. "For a person who is diagnosed with wAMD, their vision is worse –– reflecting that, their quality of life is valued at about $105,000 for a single year," she continues. "However, if they are treated with anti-VEGFs, their vision gains translate into nearly $11,000 in value after one year. Put another way, a patient with wAMD would be willing to pay $11,000 for the improved quality of life associated with better vision from the first year of anti-VEGF treatments," she summarizes.
The model reflected multiple treatment scenarios, all compared back to a "No Treatment" control group: patients in the "Less Frequent Injections" group who received approximately eight injections per year, those in the "More Frequent Injections" group who received on average 10.5 injections per year (a number closer to drug-label treatment suggestions), and several different treatment innovation scenarios to reflect the gains that might occur if more patients initiate and follow through with treatment.
After creating this model, the team used it to simulate a range of possible patient outcomes based on each scenario and compared the benefits of each outcome to its associated costs.
Their findings overwhelmingly point to the vast economic benefits anti-VEGF therapies bring to patients and society as a whole, even in light of the drugs' relatively high costs. For example, a single patient receiving less frequent injections for a five-year period can benefit from approximately $49,558 in economic gains, and a patient receiving more frequent injections during that time could witness $84,873 worth in benefits from improved quality of life.
The study further bolsters the case for increasing the number of injections per year, providing that patients respond well and experience meaningful vision gains from treatment. Their analysis shows that receiving less frequent injections accrues $873 million in population-wide value over three years while more frequent injections add $2.1 billion to societal value in three years even after accounting for the higher costs of performing more injections. Importantly, the study points out that innovations to improve treatment adherence could generate an additional $1.2 to $3.7 billion in patient benefit and $59 million to $1.3 billion in societal value compared to current treatment scenarios, highlighting the fact that when patients follow through with necessary treatment, both individuals and society as a whole can reap the rewards.
The researchers also demonstrated that if 100% of patients who needed therapy actually initiated treatment and only 6% dropped out per year (the dropout rate in clinical trial data), patients could benefit 42% more compared to simply improving treatment adherence and 89% more than they benefit from the current "Less Frequent Injections" model. In fact, in this "best case" scenario reflecting high rates of both treatment uptake and adherence, the three-year benefits could reach as high as $9.7-$15.0 billion depending on whether patients receive less or more frequent injections respectively.
Finally, the team pointed out that while politicians often scorn the high costs of anti-VEGF treatments (in 2015, Medicare Part B payed out $3.0 billion between just two anti-VEGF drugs), the team showed that relying more on a less expensive type of anti-VEGF drug known as bevacizumab could reduce costs for the full population by as much as $1.8-$2.2 billion over three years. Findings like these point to the importance of using economics to quantify the value of different therapies and auditing common practices in ophthalmology to optimize the way doctors approach treatment in a way that benefits patients and society at large.
More information: Lesley H. Curtis et al. Treatment Patterns for Neovascular Age-Related Macular Degeneration: Analysis of 284 380 Medicare Beneficiaries, American Journal of Ophthalmology (2012). DOI: 10.1016/j.ajo.2011.11.032
Eleonora M. Lad et al. Anti-VEGF Treatment Patterns for Neovascular Age-Related Macular Degeneration Among Medicare Beneficiaries, American Journal of Ophthalmology (2014). DOI: 10.1016/j.ajo.2014.05.014
Karen Mulligan et al, Economic Value of Anti–Vascular Endothelial Growth Factor Treatment for Patients With Wet Age-Related Macular Degeneration in the United States, JAMA Ophthalmology (2019). dx.doi.org/10.1001/jamaophthalmol.2019.4557