New mechanism involved in senescence modulates inflammation, response to immunotherapy

Scientists at The Wistar Institute discovered a novel pathway that enables detection of DNA in the cytoplasm and triggers inflammation and cellular senescence. This pathway may be modulated during senescence-inducing chemotherapy to affect cancer cell response to checkpoint inhibitors. Results were published online in Nature Communications.
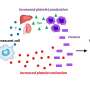
Cellular senescence is a natural tumor suppression mechanism that stably halts proliferation of damaged or premalignant cells. Senescent cells also represent a trigger of inflammation and immune reaction as they produce an array of inflammatory molecules collectively known as senescence-associated secretory phenotype (SASP).
"Uncovering an important step that mediates the senescence response and enables the SASP, we identified a novel molecular pathway involved in immunotherapy response," said lead researcher Rugang Zhang, Ph.D., deputy director of The Wistar Institute Cancer Center, professor and co-program leader of the Gene Expression and Regulation Program. "We suggest that this pathway might be targeted to modulate senescence-inducing effects of cancer therapeutics and affect response of senescent cancer cells to immunotherapy."
Cells that have been exposed to various stressors and have suffered substantial DNA damage, for example during chemotherapy, transport pieces of DNA from the nucleus to the cytoplasm as a way to signal that something is wrong. The cGMP-AMP synthase (cGAS) senses cytosolic DNA and activates senescence and immunity by triggering a cascade of cellular events that culminate with production of the SASP. How cGAS senses DNA was unknown.
To investigate this, the Zhang lab focused on the proteins attached to cytoplasmic DNA in senescent cells and identified topoisomerase 1 (TOP1) as the missing link between cGAS and DNA. TOP1 is an enzyme that unwinds the DNA helix to facilitate its replication and transcription to RNA. It has the ability to attach to DNA forming a strong DNA-TOP1 complex called TOP1cc. According to the new study, TOP1 also interacts with cytosolic DNA and cGAS, connecting the two and facilitating the DNA-sensing activity of cGAS.
Importantly, researchers also found that HMGB2, a protein that regulates chromatin structure and orchestrates the SASP at the gene expression level, enhances the interaction of TOP1 with DNA by stabilizing the DNA-bound form TOP1cc and is required for senescence and SASP.
The authors went on to establish that the HMGB2-TOP1cc-cGAS pathway is essential for the antitumor effect of immune checkpoint blockade therapy in a mouse model, as knock down of HMGB2 abated response to anti-PD-L1 treatment. Treating tumors with a TOP1 inhibitor that stabilizes the TOP1cc-DNA binding and mimics HMGB2 restored treatment response and increased survival.
"TOP1 inhibitors are clinically used for cancer therapy," said Bo Zhao, Ph.D., first author of the study and a postdoctoral researcher in the Zhang Lab. "We suggest they may have additional applications to sensitize tumors to immunotherapy, especially targeting cancer cells that become senescent in response to therapies such as chemotherapy or radiotherapy."
More information: Topoisomerase 1 cleavage complex enables pattern recognition and inflammation during senescence, Nature Communications, 2020. DOI: 10.1038/s41467-020-14652-y