Millions of products have been 3-D printed for the coronavirus pandemic—but they bring risks

With the COVID-19 pandemic, an urgent need has risen worldwide for specialized health and medical products. In a scramble to meet demand, "makers" in Australia and internationally have turned to 3-D printing to address shortfalls.
These days 3-D printers aren't uncommon. In 2016, an estimated 3% of Australian households owned one – not to mention those available in schools, universities, libraries, community makerspaces and businesses.
Across Europe and the United States, access to essential personal protective equipment (PPE) remains a concern, with nearly half of all doctors in the UK reportedly forced to source their own PPE.
In Australia, reports from March and early April showed hospital staff reusing PPE, and health-care workers sourcing PPE at hardware stores due to shortages.
The global supply chain for these vital products has been disrupted by widespread lockdowns and reduced travel. Now, 3-D printing is proving more nimble and adaptable manufacturing methods. Unfortunately, it's also less suited for producing large numbers of items, and there are unanswered questions about safety and quality control.
Sharing is caring
One of the earliest examples of 3-D printing being used for pandemic-related purposes is from mid-February. One Chinese manufacturer made 3-D-printed protective goggles for medics in Wuhan. With 50 3-D printers working around the clock, they were producing about 300 pairs daily.
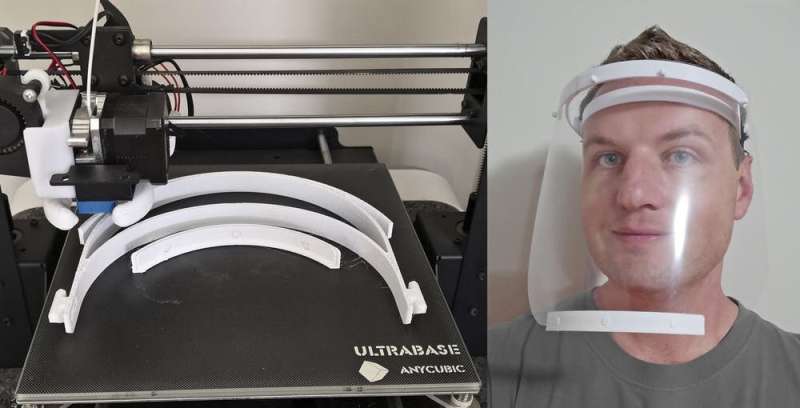
Designers, engineers, students, manufacturers, doctors and charities have used 3-D printing to produce a variety of products including face shields, masks, ventilator components, hands-free door openers and nasal swabs.
Many designs are freely shared online through platforms such as the NIH 3-D Print Exchange. This US-based 3-D printing community recently partnered with the Food and Drug Administration (FDA) and the Department of Veterans Affairs, to assist with validating designs uploaded by the community. So far, 18 3-D-printable products have been approved for clinical use (although this is not the same as FDA approval).
Such online platforms allow makers around the world not only to print products based on uploaded designs, but also to propose improvements and share them with others.
Just because you can, doesn't mean you should
In a public health crisis of COVID-19's magnitude, you may think having any PPE or medical equipment is better than none.
However, Australia's Therapeutic Goods Administration (TGA) – our regulatory body for medical products—has not yet endorsed specific 3-D-printed products for emergency use during COVID-19. Applications for this can be made by manufacturers registered with the TGA.
However, the TGA is providing guidelines which designers, engineers and manufacturers are working with. For example, Australian group COVID SOS aims to respond to direct requests by frontline medical workers for equipment they or their hospital need. So, local designers and manufacturers are directly connected to those in need.
3-D printing provides a means to manufacture unique and specialized products on demand, in a process known as "distributed manufacturing."
Unfortunately, compared with mass production methods, 3-D printing is extremely slow. Certain types of 3-D-printed face shields and masks take more than an hour to print on a standard desktop 3-D printer. In comparison, the process of "injection molding" in factory mass production takes mere seconds.
That said, 3-D printing is flexible. Makers can print depending on what's needed in their community. It also allows designers to improve over time and products can get better with each update. The popular Prusa face shield developed in the Czech Republic has already been 3-D printed more than 100,000 times. It's now on its third iteration, which is twice as fast to print as the previous version.
Opportunity vs risk
But despite the good intent behind most 3-D printing, there are complications.
Do these opportunities outweigh the risks of unregulated, untested product used for critical health care situations? For instance, if the SARS-CoV-2 virus can survive two to three days on plastic surfaces, it's theoretically possible for an infected maker to transfer the virus to someone else via a 3-D-printed product.
Medical products must be sterilized, but who will ensure this is done if traditional supply chains are bypassed? Also, some of the common materials makers use to 3-D print, such as PLA, aren't durable enough to withstand the high heat and chemicals used for sterilization.
And if 3-D-printed products are donated to hospitals in large batches, identifying and treating different materials accordingly would be challenging.
For my research, I've been tracking 3-D-printed products produced for the pandemic. In a soon-to-be-published study, I identify 34 different designs for face shields shared online prior to April 1. So, how do medical practitioners know which design to trust?
If a patient or worker is injured while wearing one, or becomes infected with COVID-19, who is responsible? The original designer? The person who printed the product? The website hosting the design?
These complex issues will likely take years to resolve with health regulators. And with this comes a chance for Australia—as a figurehead in 3-D printing education – to lead the creation of validated, open source databases for emergency 3-D printing.
This article is republished from The Conversation under a Creative Commons license. Read the original article.![]()



















