Transcriptional coregulator regulates pancreatic β-cells fitness and function
The transcriptional coregulator Swi-independent 3—or Sin3—switches on and off the genes that drive crucial biological processes during prenatal development, including cellular differentiation, maturation, survival, metabolism, and stress responses. Earlier studies reported the postnatal presence of Sin3 in the pancreas, yet its functional attributes were poorly understood.
In recent work published in Diabetes, Xiaodun Yang—from the lab of Associate Professor of Cell and Developmental Biology Guoqiang Gu—clarifies the role of Sin3 in the embryonic development and postnatal function of pancreatic β-cells, which produce insulin. Impaired β-cell function in humans accompanies reduced insulin secretion and the onset of type 1 diabetes.
On an evolutionary scale, Sin3 diverged into paralogues—Sin3a and Sin3b—two distinct forms of a single ancestral gene, each residing in a unique location within the genome, with overlapping as well as redundant functions.
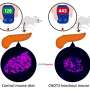
The investigators studied the roles of Sin3a and Sin3b in the pancreas both before and after birth. They found that among transgenic mice lacking Sin3a in the pancreas, Sin3a was not necessary for the prenatal differentiation of the islet cells that produce β-cells; however, it was crucial for the postnatal survival, maturation, and function of β-cells. After birth, the β-cells of the Sin3a-deletion mice failed to produce physiological levels of insulin and the mice succumbed to diabetes.
The team also found that although Sin3b-deletion mice did not show any abnormalities, those bred with neither Sin3a nor Sin3b had expedited onset of diabetes. This finding suggests redundancy in the function of both Sin3 paralogues, with Sin3a being the major contributor to β-cell function. Sin3a and Sin3b may regulate either a similar set of genes or different genes with similar functions in β-cells. In particular, the researchers identified several genes that Sin3a appeared to regulate, including some associated with protein transport, glucose metabolism, oxidative stress response, cell death, and maturation of β-cells.
Future research could leverage the stage-specific roles of Sin3a and Sin3b in the postnatal survival and fitness of β-cells to combat diabetes in humans.
More information: Xiaodun Yang et al. Coregulator Sin3a Promotes Postnatal Murine β-Cell Fitness by Regulating Genes in Ca2+ Homeostasis, Cell Survival, Vesicle Biosynthesis, Glucose Metabolism, and Stress Response, Diabetes (2020). DOI: 10.2337/db19-0721