Researchers find small mutation disrupts protein network, causing heart disease
Each day the average human heart beats 100,000 times and pumps about 2,000 gallons of blood. It's a process that is critical to life, and yet it often goes awry for unknown reasons.
"Despite knowing a lot about muscle contraction and the heart in particular, there are still a number of questions scientists have about the underlying structures and how things can go wrong, causing disease," said Bryant Chase, professor of Biological Science at Florida State University.
Chase and a team of FSU researchers have conducted a deep dive on a group of proteins and a mutation that often results in a type of heart disease called hypertrophic cardiomyopathy, a condition where the heart muscle thickens, making it difficult for the heart to pump blood. The hope is with greater understanding of the molecular underpinnings of this disease, scientists may find a path forward for treatment.
Their study, which focuses on the genetic variant of a protein called cardiac troponin C, is published in Chemical Science, a journal of the Royal Society of Chemistry.
Chase and FSU Associate Professor of Biomedical Sciences Jose Pinto have been focused on the role that troponin C protein plays in hypertrophic cardiomyopathy for several years.
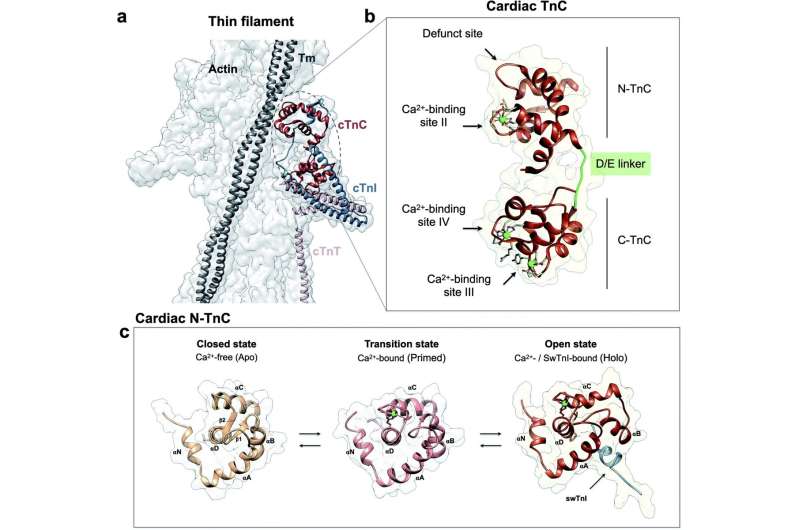
Cardiac troponin C is part of a group of related proteins that resides on filaments of striated muscle, such as the heart. It is responsible for binding calcium to activate muscle contraction—it could be thought of as an on-off switch for the heart.
The team concentrated on a genetic variant of cardiac troponin C because that variant seemed to be implicated in many cases of hypertrophic cardiomyopathy. They found that a seemingly small change in this molecule affects the protein structure so adversely that it triggers a series of problems.
"The presence of a pathogenic variant found in humans with hypertrophic cardiomyopathy in this region of cardiac troponin C disrupts the intricate network of chemical interactions within the protein. Thus, causing deleterious effects to other protein partners, negatively impacting the heart function," Pinto said.
The team conducted their research on a variety of fronts, looking at the variant in mice models while also using several advanced spectroscopy techniques to examine cellular and molecular samples. This included nuclear magnetic resonance, a technique using magnetic fields to better observe molecular changes.
They also relied on the expertise of colleagues not only from FSU, but from across the globe.
More information: Mayra A. Marques et al, Anomalous structural dynamics of minimally frustrated residues in cardiac troponin C triggers hypertrophic cardiomyopathy, Chemical Science (2021). DOI: 10.1039/D1SC01886H