Patients helping researchers to advance treatments for prostate cancer
Researchers at Monash University have established one of the world's largest collections of living tumors from prostate cancer patients, accelerating the testing of new treatments for prostate cancer and leading to faster patient benefit.
One of the most common cancers, prostate cancer is also one of the most difficult to study in the laboratory, with the frequently used models derived more than 40 years ago. With the establishment of the Melbourne Urological Research Alliance (MURAL), hundreds of Victorian men have generously donated samples of their cancer tissue, enabling the team to study a greater diversity of live tumors and test the efficacy of a larger variety of therapies for their ability to stop tumor growth.
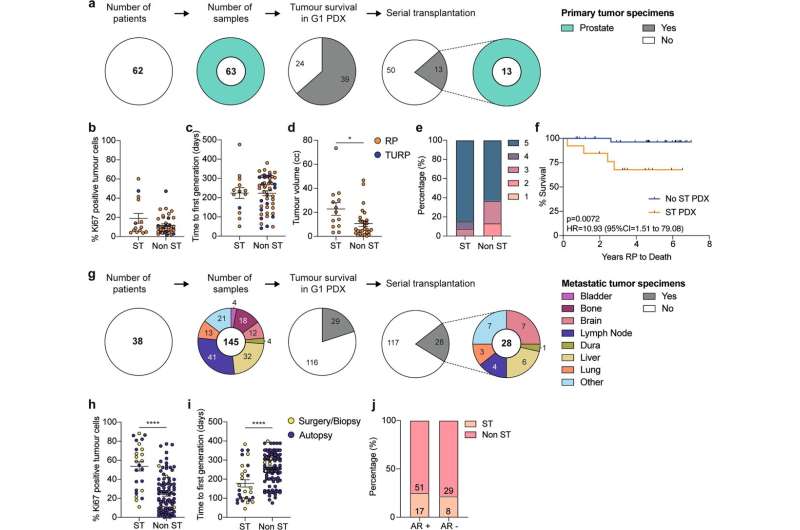
The PDX collection (patient-derived xenografts), developed by a multidisciplinary consortium and led by Professor Gail Risbridger and Associate Professor Renea Taylor at the Monash Biomedicine Discovery Institute (BDI), now comprises 59 tumors, collected from 30 patients between 2012—2020 and is now one of the largest collections of prostate cancer models in the world.
Full characterisation of the PDX collection is published in Nature Communications.
MURAL PDXs are an enduring resource of new cancer models that can be shared with other academic investigators or pharmaceutical companies. The patients and their families are directly embedded in this venture, including the EJ Whitten Foundation who have been pivotal over the last 10 years in providing over $1M in donations enabling this resource to be developed and the program to come to the forefront of the international field.
"This project begins and ends with patients like EJ Whitten. We take patient tissue—do testing in the laboratory—and the discoveries then advance treatment for patients," said Professor Risbridger. "Our new models of prostate cancer have attracted interest from scientists and the pharmaceutical industry worldwide."
Ted Whitten, executive director and founder of the E.J.Whitten Foundation congratulates the Monash University Biomedicine Discovery Institute on its recent findings in regards to Prostate Cancer research. "We believe that Monash University is a leader of Prostate Cancer Research and we have been delighted to have been able to financially support many of their important programs over the past ten years."
Dr. Mitchell Lawrence, also from Monash BDI and a senior author, says: "This resource provides an opportunity to link the molecular changes in prostate cancer to pathology, grow organoids and test functional responses to therapies, which have rarely been applied to prostate cancer given the lack of suitable models."
More information: Gail P. Risbridger et al, The MURAL collection of prostate cancer patient-derived xenografts enables discovery through preclinical models of uro-oncology, Nature Communications (2021). DOI: 10.1038/s41467-021-25175-5