Highly accurate point-of-care neutralizing antibodies (NAb) test for COVID-19
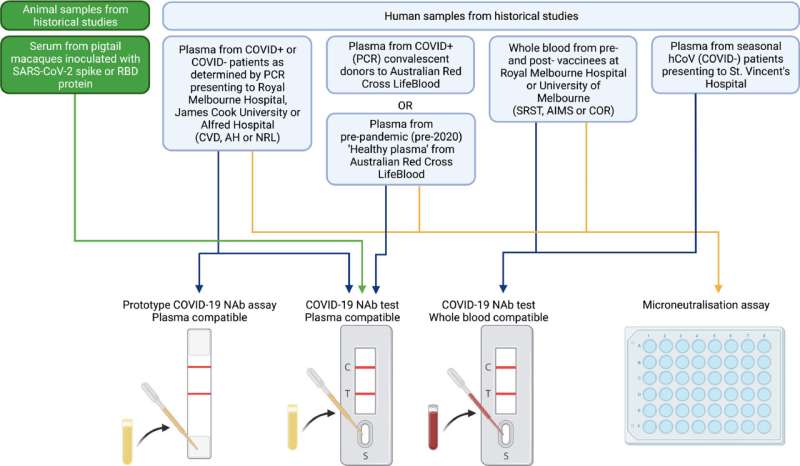
Staying one step ahead of COVID-19 variants is one of the potential benefits of a new, highly accurate, point-of-care (POC) test that can measure the level of neutralizing antibodies (NAb) to COVID-19—including variants—developed by Burnet Institute and the Peter Doherty Institute for Infection and Immunity (Doherty Institute) scientists.
The test, which provides a readout from a fingerprick of blood in less than 20 minutes, is the first of its type globally, and may also be useful to indicate when a vaccine booster may be required.
While there are many POC tests that can measure the overall level of antibody to SARS-CoV-2, and use this to estimate neutralizing antibodies, this is the only test to measure neutralizing antibody activity, which correlates with immune protection from serious disease in COVID-19.
The emergence of the Omicron variant, which early studies suggest may be up to 40-times less sensitive to patient NAb than Delta, means that some people will go from having enough NAb to be protected, to not having enough.
Being able to measure this will be important:
- to prioritize additional booster shots to those who don't have enough immunity against Omicron or future variants including people at risk of severe disease—the elderly and people with co-morbidities, and people at high risk of exposure such as frontline healthcare workers
- to check that these people have responded to their vaccine or booster
- to provide a standardized test to assist in the safe reopening of borders in the context of different vaccines and the variable responses in different populations.
The test provides a new option for immunity screening to support COVID-19 vaccination and control programs, particularly in time-critical and low- and middle-income settings where laboratory-based testing is difficult or impossible for many to access.
"One of the key elements of the test is that it can work with fingerprick whole blood, which is essential if it is really going to be used at point-of-care," study joint supervising author, Associate Professor David Anderson, Burnet Institute Deputy Director and Chief Scientific Officer of the Burnet Diagnostics Initiative, said.
"We have demonstrated that we can readily substitute different variants into the test. While we have not yet tested Omicron, this will be simple enough to incorporate in our test in place of the original strain or other variants that we have tested so far."
Co-first author, Burnet Senior Development Scientist Huy Van, said a successful collaboration was fundamental to the development of the test.
"I have worked on a lot of different point-of-care tests over the years, but the COVID-19 NAb Test is probably the one where we had the greatest number of different complications to overcome in making a simple test that works," Mr Van said.
"But it's also been the best collaboration between groups with very different areas of expertise and resources across the Burnet and Doherty Institutes to achieve that in such a short time."
Dr. Tom Fulford from the Doherty Institute said: "We envisage that this test may be part of a set of indications that will help to determine who may need a booster vaccine.
"This can also be adapted so that it can provide information about whether an individual has protective antibodies against particular variants of concern.
"This has been a fantastic collaborative effort between a number of labs at the Doherty Institute, at the Royal Melbourne Hospital and our colleagues at the Burnet Institute, bringing together some of the best scientists in Australia to develop a test we hope will help manage our response to COVID-19."
Currently in the prototype phase, the Institutes are currently seeking commercial partners to develop the manufacturing process and take the test to the global market.
Jen Barnes, director of the Burnet Diagnostics Initiative (BDI), said the collaboration has led to a novel lab prototype in record time, and is an example of the driver behind the formation of the BDI.
"The BDI aims to enhance the translation of new technologies to practical health solutions through significant partnerships with academic collaborators and industry. The NAb test is a great example of this as we look for a global partner to bring the test to market," she said.
This study, published in the journal EBioMedicine, was supported by funding from the Victorian Government.
More information: Thomas S. Fulford et al, A point-of-care lateral flow assay for neutralising antibodies against SARS-CoV-2, EBioMedicine (2021). DOI: 10.1016/j.ebiom.2021.103729