Immune response may vary with choice of COVID-19 booster

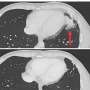
(HealthDay)—Immune response may vary with the choice of COVID-19 vaccine used for a third dose or booster following two doses of ChAdOx1 nCoV-19 (ChAd) or Pfizer BioNTech BNT162b2 (BNT), according to a study published online Dec. 2 in The Lancet.
Alasdair P. S. Monro, M.D., from the University Hospital Southampton NHS Foundation Trust in the United Kingdom, and colleagues conducted a blinded, multicenter, randomized, controlled phase 2 trial of third-dose booster vaccination against COVID-19. Participants were older than 30 years and were at least 70 days since two doses of ChAd or at least 84 days since two doses of the BNT primary COVID-19 vaccination course. Eighteen sites were split into three groups (A, B, C): Within each site group, participants were randomly assigned to an experimental vaccine or control. Group A received Novavax NVX-CoV2373 (NVX), a half dose of NVX, ChAd, or quadrivalent meningococcal conjugate vaccine (MenACWY) control; Group B received BNT, Valneva VLA2001 (VLA), half dose VLA, Janssen Ad26.COV2.S (Ad26), or MenACWY; and Group C received Moderna mRNA-1273 (m1273), CureVac CVnCOV (CVn), a half dose of BNT, or MenACWY.
A total of 2,878 participants received COVID-19 vaccine or control. The researchers found that three vaccines showed overall increased reactogenicity: m1273 after ChAd/ChAd or BNT/BNT, and ChAd and Ad26 after BNT/BNT. For ChAd/ChAd-primed individuals, the spike immunoglobulin G (IgG) geometric mean ratios (GMRs) varied from 1.8 in the half-VLA group to 32.3 in the m1273 group. For BNT/BNT-primed individuals, spike IgG GMRs varied from 1.3 to 11.5 for half VLA and m1273, respectively.
"Whilst all boosted spike protein immunogenicity after two doses of AstraZeneca, only AstraZeneca, Pfizer-BioNTech, Moderna, Novavax, Janssen, and CureVac did so after two doses of Pfizer-BioNTech," a coauthor said in a statement.
Several authors disclosed financial ties to biopharmaceutical companies, including AstraZeneca and Pfizer.
More information: Abstract/Full Text
Copyright © 2021 HealthDay. All rights reserved.