Research clears up how brain 'cleaners' fail in ALS
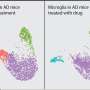
In preclinical studies, Mayo Clinic scientists and collaborators have identified the molecular mechanism used by the brain's "cleaners" as they remove a problematic protein in the brain. This work, published in Nature Neuroscience, demonstrates that the cleaners—resident immune cells in the brain called microglia—play a protective role in a mouse model of Lou Gehrig's disease, also known as amyotrophic lateral sclerosis (ALS). The study may provide a potential therapeutic target for that disease.
The loss of neurons characterizes the neurodegenerative diseases of Alzheimer's and ALS. In Alzheimer's, the lost neurons handle memory retrieval, and in ALS, it is the neurons that manage movement that are damaged. In the brain of people diagnosed with either of these diseases, pathologists can see a buildup of specific proteins: beta-amyloid and tau in those with Alzheimer's, and a protein called TAR-DNA binding protein 43 kDa (TDP-43) in people with ALS.
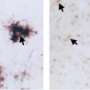
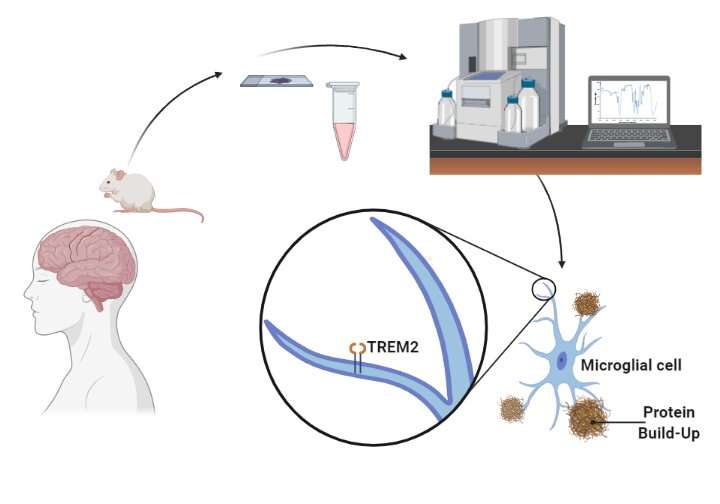
Microglia are the cells tasked with cleaning up debris in the brain, and they have a unique receptor called TREM2. When this receptor is mutated, evidence suggests risks increase for developing Alzheimer's disease, the theory being that microglia cannot clean up the brain efficiently. But the evidence for TREM2 and ALS was more tenuous until now.
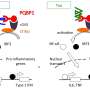
"The aggregation of TDP-43 in the brain is a hallmark for most ALS patients," explains Long-Jun Wu, Ph.D., a Mayo Clinic neuroimmunologist and senior author of the paper. "Our study shows for the first time that TDP-43 is a potential ligand for microglial TREM2. Further, we found that this interaction mediates microglial TREM2 sensing and clearance of pathological TDP-43 protein.
As a ligand, TDP-43 binds to the TREM2 receptor, which is important for microglia cells to clear up the protein. Using biochemistry, computational simulations, confocal microscopy, mouse models, and samples from the Mayo Clinic Brain Bank for Neurodegenerative Disorders, the researchers were able to decipher the interaction between TREM2 and TDP-43, thereby potentially revealing a target for therapy for ALS.
"The mechanisms underlying ALS initiation and progression are poorly understood," says Dr. Wu. "Microglia comprise a unique subset of glial cells and are the principal immune cells in the central nervous system. Our current findings point out microglial TREM2 as a potential therapeutic target for ameliorating TDP-43-related neurodegeneration, including ALS."
The Neuroimmune Interaction in Heath and Disease Laboratory led by Dr. Wu plans to delve into the exact binding sites of human TDP-43 and the TREM2 receptor. They also want to investigate a specific population of microglia that seem to be supercharged to remove TDP-43. And the eventual goal is to explore if TREM2 activators might be a candidate for treatment in mouse models of ALS, which is a first step toward potentially treating human disease.
More information: Manling Xie et al, TREM2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration, Nature Neuroscience (2021). DOI: 10.1038/s41593-021-00975-6