New opportunities for targeting overactive cancer genes
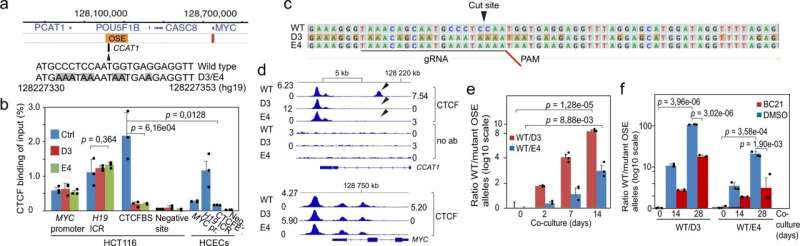
Anita Göndör's group has identified a new mechanism underlying the pathological over-expression of cancer genes. The results, which are published in Nature Communications show that signals in the environment of the cancer cell exploit a CTCF-bound super-enhancer to recruit cancer genes to the nuclear pore in a multistep process that involves a non-coding RNA. This discovery provides new opportunities for targeting overactive cancer genes without interfering with their functions in normal cells.
A gene called MYC is central for normal cell proliferation. If it is over-activated, however, pathological cell proliferation will ensue. It is well known that the so-called oncogenic super-enhancers have the capacity to sustain a high level of target gene expression, including MYC, in tumor cells. The research group has earlier shown that such a super-enhancer is able to recruit the active MYC gene to nuclear pores and, in this way, increase its expression. However, the mechanistic underpinnings of this principle were not known before.
The current study shows that an overactive WNT signaling pathway, a well-known feature of many cancer cells, ensures that a CTCF binding site within the oncogenic super-enhancer that regulates MYC recruits protein factors known to be pivotal for the nuclear export of mRNA. Moreover, in a step-wise process that includes the activation of a local non-coding RNA (CCAT1), the CTCF-super-enhancer complex gradually brings the active MYC gene to nuclear pores to facilitate the nuclear export of the MYC mRNAs. Since the MYC mRNA is more stable in the cytoplasm than in the nucleus, this entire process ensures a several-fold increase in total cellular MYC expression.
By using drugs that neutralize WNT signaling, the research team could block this entire process without affecting the expression levels of MYC in normal cells.
"Our results provide a surprising and completely novel perspective on the mechanisms underlying the unscheduled expression of cancer genes. Since this principle was specific for colon cancer cells and not active in normal colon epithelial cells, it will likely open up new avenues for reducing uncontrolled cancer cell proliferation by using more targeted drugs," says the leader of the research team, Anita Göndör who is an associate professor at the Department of Oncology and Pathology at Karollinska Institutet.
The next step will be to identify new drug combinations that target the dynamics of the stepwise trafficking process. The aim is to pave the way for new drug combinations that have less side effects than radiation and chemotherapies.
"The strength of our study is that it has identified key players underlying the cancer-specific over-expression of a well known and targetable protein that drives pathologic gene expression, providing new angles for more efficient and specific treatment of cancer patients," says Anita Göndör.
More information: Ilyas Chachoua et al, Canonical WNT signaling-dependent gating of MYC requires a noncanonical CTCF function at a distal binding site, Nature Communications (2022). DOI: 10.1038/s41467-021-27868-3



















