Promising new treatment for dry eye disease
The University of Manchester, Seoul National University College of Medicine and Link Biologics Limited today announced that promising preclinical data on the treatment of dry eye disease using a novel protein biological drug, Link_TSG6, have been published in the peer-reviewed journal Ocular Surface.
Dry eye disease (DED) is the most common ocular surface disorder, affecting approximately 350 million people worldwide and causing persistent eye irritation, blurred vision, pain and decreased quality of life. DED is characterised by a loss of homeostasis of the eye's tear film and a vicious cycle of corneal epithelial damage and inflammation.
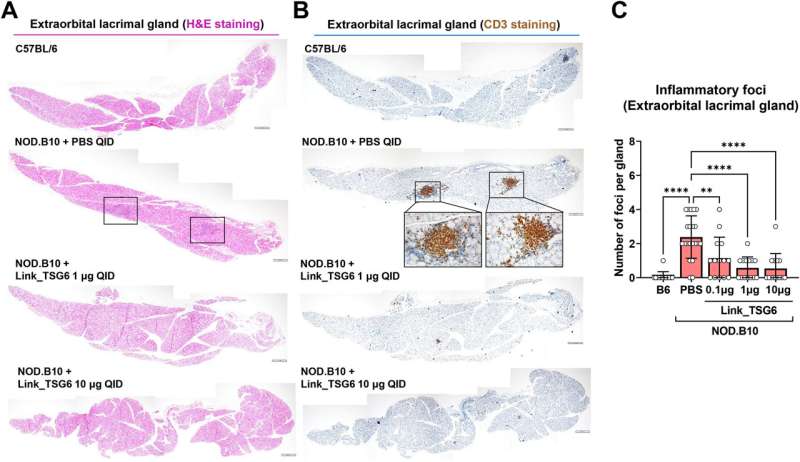
The newly-published study evaluated Link_TSG6 in two validated mouse models of DED: an autoimmune model where NOD.B10.H2 mice spontaneously develop dry eye disease, and the desiccating stress model that mimics DED caused by environmental factors. Results from the study showed that topically administered Link_TSG6 (e.g., twice a day for 7 days) dose-dependently reduced corneal epithelial defects and suppressed inflammatory markers while increasing tear production and conjunctival goblet cell density. At the highest Link_TSG6 dose, no corneal lesions remained in approximately 50 percent of treated eyes. In addition, Link_TSG6 was significantly more effective than Restasisâ, the market leading treatment, at reducing corneal epithelial erosions and reducing inflammation.
"There is a desperate need for treatments that rapidly and significantly improve the signs and symptoms of dry eye disease," said Dr. Joo Youn Oh from Seoul National University College of Medicine, a corresponding author of the paper. "The present findings support the viability of Link_TSG6 as a promising drug candidate that both suppresses inflammation and promotes repair of the cornea—critical steps in overcoming the pathophysiology of dry eye disease."
"Link_TSG6 is a biological drug that harnesses the protective effects of TSG-6, a protein that is made in our bodies in response to inflammation and injury. It is extremely rewarding to obtain such compelling preclinical data with our novel treatment approach for dry eye disease. We hope to further progress this work by advancing Link_TSG6 towards human clinical trials. It is our ambition to see Link_TSG6 approved and available to patients with DED," says Professor Tony Day.
The study showed that Link_TSG6 suppresses the levels of inflammatory cytokines on the ocular surface and inhibits the infiltration of Th1 and Th17 immune cells into the lacrimal glands and lymph nodes, indicative of the protein's multiple anti-inflammatory effects.
Professor Tony Day, a co-corresponding author, from the Wellcome Centre for Cell-Matrix Research, University of Manchester, said: "Link_TSG6 is a biological drug that harnesses the protective effects of TSG-6, a protein that is made in our bodies in response to inflammation and injury. It is extremely rewarding to obtain such compelling preclinical data with our novel treatment approach for dry eye disease. We hope to further progress this work by advancing Link_TSG6 towards human clinical trials. It is our ambition to see Link_TSG6 approved and available to patients with DED."
Caroline Milner, a Founder of Link Biologics, said: "The publishing of this research is a major accomplishment in our journey towards finding an effective treatment for dry eye disease, which could improve the lives of millions around the world. The team is working diligently to build on these findings and secure additional funding to reach our next milestone."
More information: Joo Youn Oh et al, The Link module of human TSG-6 (Link_TSG6) promotes wound healing, suppresses inflammation and improves glandular function in mouse models of Dry Eye Disease, The Ocular Surface (2021). DOI: 10.1016/j.jtos.2021.12.012