Method enables blood-brain barrier repair in neurological disorders
![Repurposing Wnt7a ligands into BBB therapeutics.BBB dysfunction has been implicated in the etiology of a large set of CNS disorders. Wnt7a/b ligands, which dominate the neurovascular differentiation cascade during vertebrate development, are here repurposed as safe BBB therapeutics by engineering them into highly specific Gpr124/Reck agonists. [Illustration created with BioRender]. Credit: DOI: 10.1126/science.abm4459 Method enables blood-brain barrier repair in neurological disorders](https://scx1.b-cdn.net/csz/news/800a/2022/method-enables-blood-b.jpg)
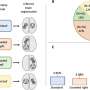
An impressive number of brain pathologies are closely linked to major cerebrovascular defects, which are currently impossible to treat due to a lack of drugs. The discovery by researchers from the ULB Neuroscience Institute is therefore particularly promising, as not only have they developed a new class of molecules that specifically correct these dysfunctions, they have also demonstrated their effectiveness in mouse models of radically different brain pathologies.
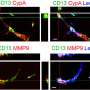
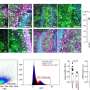
Led by Prof. Benoit Vanhollebeke—WELBIO investigator and professor at the Department of Molecular Biology, Faculty of Sciences, Free University of Brussels—the researchers at the Neurovascular Signalling Laboratory, ULB Neuroscience Institute, specialize in the study of cerebral blood vessels and their dysfunction. By studying the proteins controlling the formation of these vessels during embryonic life, the researchers believe they can identify targets with promising therapeutic potential. The evidence is that by developing molecules targeting the Gpr124/Reck membrane complex, whose role was first revealed in a neurodevelopmental context, Maud Martin and her colleagues have succeeded in slowing down the progression of glioblastoma, the most common primary adult brain cancer, in mice, and reducing the lesions following a stroke.
The same team had previously published on the mechanistic characterisation of the therapeutic target in Science. This new study establishes its therapeutic potential in mice. When the target is activated, dysfunctional cerebral blood vessels made too permeable by the pathology regain their original functionality; they recover a set of cellular and molecular characteristics that strongly limit exchanges between blood and neural tissue and are collectively called the blood-brain barrier. The brain is thus again protected from toxic components circulating in the blood, and the progression of pathologies is slowed.
"One of the most fascinating aspects of this study is the level of specificity with which pathological brain vessels respond to this experimental treatment. Inspired by the natural developmental process, we have designed a new class of molecules that are able to reach their therapeutic target efficiently, while remaining completely inert for healthy vessels and other tissues of the body. On a fundamental basis, this level of specificity seemed a priori out of reach," explains Benoit Vanhollebeke.
To build on this, the researchers from the Neurovascular Signalling Laboratory now want to explore other experimental models of brain pathologies that could potentially benefit from their approach.
Benoit Vanhollebeke and the ULB have created the spin-off company NeuVasQ Biotechnologies, which, with the support of a consortium of public and private investors, aims to bring this type of neurovascular treatment to the bedside.
More information: Maud Martin et al, Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders, Science (2022). DOI: 10.1126/science.abm4459